Abstract
Objective
We conducted an observational study to investigate changes in symptoms and their degree by bathing in asthmatic patients.
Methods
A questionnaire focusing on ever experienced bathing-induced symptom changes and their degree, as well as contributing factors, was designed and administered to asthmatic patients in the outpatient department of our institute between January 2012 and November 2013.
Results
Two hundred fifteen cases were recruited. In 60 cases (27.9%), asthmatic symptoms appeared, including 20 cases of chest discomfort (33.3%), 19 cases of cough (31.7%), and 21 cases of wheezing (35.0%). The triggering factors included vapor inhalation (32 cases, 53.3%), hydrostatic pressure on the thorax due to body immersion in the bathtub (26 cases, 43.3%), and sudden change of air temperature (16 cases, 26.7%). Thirty-eight cases (17.7%) experienced improvement in active asthmatic symptoms by bathing. Vapor inhalation was the most common contributing factor (34 cases, 89.5%), followed by warming of the whole body (13 cases, 34.2%). There was no relationship between asthma severity and the appearance of bathing-induced symptoms or improvement of active asthmatic symptoms by bathing.
Conclusion
The effects of bathing in asthmatic patients widely differed from patient to patient and their etiology includes several factors. For those who suffer from bathing-induced asthma symptoms, preventive methods, such as premedication with bronchodilators before bathing, should be established. This study is registered in the University Hospital Medical Information Network (UMIN) clinical trials registry in Japan with the registration number UMIN000015641.
Bathing is closely related to daily life but its effects on the symptoms of asthma have not been investigated in detail. The triggering factors for asthma deterioration due to bathing include "vapor inhalation during bathing" in Japanese guidelines for asthma [1]. The suggested mechanism is bronchoconstriction due to stimulation from hypo-osmolar aerosol deposits in the airway [23]. On the other hand, some patients do experience improvement of symptoms by bathing mainly due to inhalation of the vapor. Mist inhalation therapy for asthmatic children results in either improvement or deterioration [4]; thus, the response from bathing differs from patient to patient.
"Paradoxical bronchoconstriction (PBC)" is a phenomenon which is caused by the administration of bronchodilators via nebulizer, resulting in unexpected bronchoconstriction [5]. The suggested mechanism includes airway stimulation due to changing osmolarity in the bronchus, preservatives, or stabilizers in nebulizer solutions [56]. Deterioration from daily bathing, namely bathing-induced asthma (BIA), and PBC may share the same mechanism: bronchial stimulation from excessive vapor deposits in the airway.
The purpose of this study is to investigate the effects of daily bathing on the symptoms of asthmatic patients and analyze the causative factors. In addition, we investigated the relationship between history of BIA and that of PBC.
Two hundred fifteen patients with physician-diagnosed asthma who visited the outpatient department of the National Hospital Organization Disaster Medical Center, Tokyo, Japan, between January 2012 and November 2013 were included in this study.
An observational study based on a questionnaire focusing on ever experienced bathing-induced symptom changes, was conducted. The questionnaire included the following items: Current treatment steps based on the GINA guidelines [7] (Steps 1, 2, 3, 4, 5); Effects on asthmatic symptoms by bathing (have ever experienced deterioration, have ever experienced improvement during the undercontrol phase of asthma, never experienced any changes); If there was experiences of deterioration, emerged symptoms from which the patients have suffered most (chest discomfort, cough, wheezing), possible causes (vapor inhalation, sudden change of air temperature, hydrostatic pressure generated by body immersion up to the shoulders in the bathtub, hyperthermia, other causes), and condition of asthma status when the symptoms deteriorate by bathing (only when asthma is undercontrol, even when asthma control is good); If there was experiences of improvement, extent of symptom improvement (partial, complete) and possible factors (vapor inhalation, hyperthermia, enhanced sputum expectoration, other factors); and History of PBC, paradoxical deterioration of asthma symptoms including wheezing or cough by administration of β2-agonist via nebulizer (yes, no, no history of nebulizer use).
Based on the results of the questionnaire, the incident rates of history of deterioration or improvement of asthmatic symptoms and their degree by bathing, and causative factors for these symptom changes were estimated. Those who have the history of deterioration were defined as the cases of BIA. The distribution of bathing-induced symptom changes and their degree, and causative factors among each treatment step was statistically analyzed by using corresponding analysis. Among those who had a history of nebulizer use with β2-stimulant for asthma deterioration, the incident rate of PBC and its relationship with BIA and causative factors for BIA were estimated. Additional use of mucolytics such as bromhexine was not used in the nebulizer solution. The relationship was statistically estimated by using Fisher exact test. Calculations were performed using the JMP for Windows version 10 (SAS Institute Inc., Cary, NC, USA). All p values were two-tailed with a p value <0.05 considered statistically significant.
This study was approved by the ethics committee of the National Hospital Organization Disaster Medical Center (registration number 2012-5) and verbal informed consent was obtained from all participants in the study. This study is registered in the University Hospital Medical Information Network (UMIN) clinical trials registry with the registration number UMIN000015641.
Out of 215 patients (women 63.7%; median, 65 years; range, 21 to 95 years), 60 patients (27.9%) had the experience of deterioration of symptoms by daily bathing, and 38 (17.7%) had the experience of improvement (Table 1). The patients in deterioration group and improvement group experienced their episodes repeatedly and were mutually exclusive. Steps 1 (n = 3) and 2 (n = 30) were combined into Steps 1 + 2, since the number of step 1 is very small. No statistical difference was found between the severity of asthma and effects of bathing. (p = 0.285, corresponding analysis).
Major causative factors of symptom deterioration in the 60 patients included vapor inhalation (n = 32, 53.3%), hydrostatic pressure on the thorax due to body immersion into water up to their shoulders (n = 26, 43.3%), and exposure to cold air, such as undressing or cold air inhalation before the bating during the winter season (n = 16, 26.7%) (Table 2). Minor factors included hyperthermia (n = 2, 3.3%) and a chilly feeling after a bath, anteflexion posture while washing the hair, and unknown cause in each one case. No relationship was statistically found between causative factors and asthma severity (p = 0.564, corresponding analysis). Emerging symptoms by bathing included chest discomfort in 20 patients (33.3%), cough in 19 patients (31.7%), and wheezing in 21 patients (35.0%). No relationship was statistically found between emerging symptoms and asthma severity (p = 0.552, corresponding analysis). In 38 patients (63.3%), deterioration of symptoms occurred only when asthma is undercontrol and in 22 patients (36.7%) symptoms emerged even when their asthma control was stable. More patients experienced deterioration of their symptoms by bathing during undercontrolled periods. No relationship was statistically found between the condition of asthma control at the time of BIA and asthma severity (p = 0.128).
Twelve patients suffered from BIA at the time of the survey and administration of β2-stimulant before bathing was conducted as a preventive method. Inhalation of short-acting β2-agonist (SABA) was performed in 3 patients and BIA was successfully prevented. Regular inhalation of combined inhaled corticosteroid (ICS) and long-acting β2-agonist (LABA) was shifted from after to before daily bathing in 9 patients, and BIA was prevented in 8 patients and not prevented in 1 patient, who had the most severe grade of asthma.
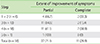
Major factors for symptom improvement in the 38 patients included vapor inhalation (n = 34, 89.5%), and hyperthermia (n = 13, 34.2%) (Table 3). Minor factors included sputum expectoration in 3 patients (7.9%) and unknown in 1 (2.6%). In 11 patients, the symptom of wheezing disappeared by bathing completely, and in 27 patients, symptoms improved but did not completely disappear. No relationship was statistically found between the extent of symptom improvement and asthma severity (p = 0.477, corresponding analysis). No relationship was statistically found between improvement factors and asthma severity (p = 0.963, corresponding analysis).
Out of 215 patients, 152 had a history of administration of nebulized bronchodilators. Out of these 152 patients, 45 (29.6%) experienced deterioration of symptoms by bathing and 13 (8.6%) experienced PBC. PBC was seen in 9 patients (20.0%) of the deterioration group, in 2 patients (7.7%) of the improvement group, and in 2 patients (2.5%) of the no change group. The severity of these 13 patients belonged to steps 3 to 5, and mostly occurred in female patients (n = 12, 92.3%). PBC occurred more frequently in the deterioration group compared to the no change group (Fig. 1, p = 0.002). Out of the 9 patients who experienced PBC in the deterioration group, the triggering factor for deterioration by bathing was vapor inhalation in 8 patients (88.9%). However, 2 patients whose asthmatic symptoms improved by vapor inhalation during bathing also experienced PBC.
In 45 patients of the deterioration group, vapor inhalation was the causative factor in 25 patients, and was significantly correlated with PBC (p = 0.031). Other factors, such as hydrostatic pressure, change of air temperature, and hyperthermia, were not correlated with PBC (Table 4). Though the frequency of PBC in the 45 patients in the deterioration group did not show a statistical significance compared to that of the improvement group (Fig. 1, p = 0.307, Fisher exact test), in the subgroup of 25 patients whose causative factor for BIA was vapor inhalation, the frequency of PBC was significantly higher than that of the improvement group (p = 0.039).
In asthmatic patients, 27.9% experienced deterioration of symptoms, whereas 17.7% experienced improvement of symptoms by daily bathing. The patients in deterioration group and improvement group experienced their episodes repeatedly and were mutually exclusive, thus bathing-induced deterioration or improvement could be regarded as consistent phenotypes. The same stimulation resulted in various responses among the patients, which suggests the diversity of bronchial asthma. Moreover, bathing involves several kinds of stimuli, mainly vapor inhalation, hydrostatic pressure on the thorax, exposure to cold air, and hyperthermia, all at the same time; thus, BIA is the result of the composite of these stimuli. The effects in asthmatic patients by each factor, vapor inhalation [89], exposure to cold air [1011], and hydrostatic pressure on the thorax [12], have been investigated previously, but the effects of bathing itself, a composite of these stimuli, have never been reported. There is a cultural aspect and the effects of bathing might differ according to bathing style. In general, compared to Western bathing, Japanese bathing involves a longer bathing time, hotter water temperature, and deeper bathtub resulting in higher hydrostatic pressure on the thorax [13]. Thus, Japanese bathing is prone to result in stronger airway stress for asthmatic patients than Western bathing.
The most frequent cause of deterioration of symptoms was vapor inhalation. The mechanisms of bronchoconstriction by vapor inhalation include airway stimulation due to a change in osmolar pressure, which causes enhanced degranulation from inflammatory cells and enhanced vagal nerve stimulation [12]. The elevation of serum histamine concentration and neutrophil activation has been reported in patients in whom bronchoconstriction was provocated by inhalation of water vapor [8]. In addition, inhalation of hot air with high humidity stimulates cholinergic nerve reflex, C-fiber, and TRPV1 (transient receptor potential cation channel subfamily V member 1), resulting in bronchoconstriction [14], and the degree of bronchoconstriction is twice as much as that induced by cold air inhalation [15]. As a preventive method of these phenomena, premedication by ipratropium bromide or sodium cromoglycate has been shown to prevent bronchoconstriction induced by inhalation of the mist of distilled water [16]. Similarly, in this study, premedication by bronchidilators prevented the emergence of BIA symptoms.
PBC has been reported since 1966 [17]. Nebulizer inhalation can stimulate the airway via excessive particle deposition, change in osmolar pressure [818], low pH of drugs, and preservatives or propellants in drugs [51920]. A common mechanism underlies BIA and PBC, and this study revealed a close relationship between these two conditions. Thus, close attention should be paid to those with BIA when SABA via nebulizer is applied during asthma exacerbation. PBC was not only seen in BIA patients but also in the improvement group. This might be explained by the fact that preservatives or acidity of the nebulizer solution might act as irritants for the airway. Or possibly in some patients, a moderate amount of vapor will act as a bronchodilator, whereas excessive vapor will act as an airway stimulant.
Other factors in the deterioration group included hydrostatic pressure on the thorax due to body immersion and exposure to cold air as major causes, and hyperthermia as a minor cause. Body immersion up to the shoulder level results in a 25% reduction of functional residual capacity [21], 10% reduction of vital capacity [2122], and 58% increase in airway resistance [21] by hydrostatic force and elevation of the diaphragm in healthy adults. Thus, in asthmatic patients, subclinical airway obstruction could be clinically symptomatic during bathing. Cold air inhalation [2324], or even body cooling [252627], is also known to stimulate the airway in asthmatic patients; especially in the winter season, the physiological burden to asthmatic patients might be high owing to the cold climate.
Rodriguez et al. [4] conducted a mist inhalation challenge in 34 asthmatic children and two-thirds of the patients showed either improvement or deterioration. In our experience, less than 20% of patients experienced improvement by bathing, and vapor inhalation was the greatest factor. The protective effect of warm humid air inhalation against bronchoconstriction in EIA [28] or body cooling [26] has also been reported, but the positive effects in asthmatic patients of vapor inhalation has been far less investigated compared to the negative effects. The mechanism of improvement by mist inhalation is thought to involve better mucous clearance due to humidification of the airway. Hyperthermia was the second greatest factor for symptom improvement. Increased body temperature is known to reduce the effect of platelet-activating factor on airway responsiveness [29]; thus, it might act as a factor for bronchodilation. For those whose symptoms improved by these factors, inhalation of nebulized mist or body warming might be considered as supplemental therapy when symptoms cannot be controlled despite standard therapies.
The evaluation of the effects induced by bathing was based on patients' impressions and memories, and there was a lack of objective parameters, such as peak flow rate or spirometers. Concerning about the symptoms for BIA, the patients were asked to choose the most suffering symptom among three ones. Naturally 2 or 3 symptoms can coexist, but we thought that it would be better to count only major symptom than to count both major and minor symptoms as one same weight. For more precise evaluation, quantification of each symptom should have been made. The patients were exposed to several kinds of stresses at the same time, and so their perception of the possible causes of deterioration or improvement might be different from the real causes. Inhalation of hot air and hyperthermia might be indistinguishable for some patients. Likewise, the responsible cause might be perceived as a single factor, though the reality might be multifactorial. For example, inhalation of hot vapor would cause bronchoconstriction while inhalation of room temperature vapor might not. To investigate the real causes in each case of BIA or improvement by bathing, a challenge test for each factor (vapor inhalation, water pressure, sudden change of air temperature, hyperthermia) might be needed, as well as different combinations of each factor; however, this may not be feasible to perform. Moreover, the condition of bathing including the temperature and moisture of bath room and the living environment might be different among the study population, and if these conditions were controlled as same level, the rate of symptoms change might be different. This study simply revealed the overall response of multi-factorial stress by bathing in asthmatic patients in different conditions of bathing environment and importantly proved that BIA might be prevented by premedication with β2-agonist.
In this study, no relationship was found between the incidence of BIA and asthma severity. The study was conducted in a tertiary referral hospital and the number of patients with mild intermittent or mild persistent asthma was limited. Thus, the results of incidence of BIA or its relation to asthma severity might be different when these kinds of patients are recruited in sufficient numbers.
Though bathing is closely related to our daily life and an unavoidable activity, BIA has been far less investigated compared to daily activity-induced asthma, such as exercise-induced asthma (EIA). As in the case of premedication by inhalation of SABA or cromoglycate for EIA [3031], a preventive method should be established for BIA. Formoterol is known to act quickly after inhalation [3233] and salmeterol within 10 [34] to 30 minutes [32]; thus, by shifting the timing of regular inhalation of ICS/LABA to before bathing, BIA can be controlled without increasing or adding medications, and should be considered as one of the inhalation methods. The long-term effects of asthma control by preventive therapy on BIA should be investigated since patients are exposed to offending factors every day.
For those who improve by bathing, airway humidification as a supplemental therapy should be considered for better asthma control, and long-term effects should also be investigated.
In conclusion, in asthmatic patients, the effects of daily bathing widely differed from improvement to deterioration. Inhalation of vapor was the most frequent contributing factor in both the deterioration and improvement groups. BIA and PBC were closely related, both involving airway stimulation by vapor inhalation as the common underlining mechanism. Preventive methods, such as inhalation of β2-agonist before bathing, should be established for BIA.
Figures and Tables
ACKNOWLEDGEMENTS
The authors received no financial support and have no conflict of interest to declare in association with this work.
References
1. Asthma Prevention and Management Guideline 2015' Creating committee. Asthma Prevention and Management Guideline 2015. Tokyo: Kyowakikaku;2015.
2. Smith CM, Anderson SD. Inhalation provocation tests using nonisotonic aerosols. J Allergy Clin Immunol. 1989; 845(Pt 1):781–790.

3. Anderson SD, Smith CM. Osmotic challenges in the assessment of bronchial hyperresponsiveness. Am Rev Respir Dis. 1991; 1433(Pt 2):S43–S46.

4. Rodriguez GE, Branch LB, Cotton EK. The use of humidity in asthmatic children. J Allergy Clin Immunol. 1975; 56:133–140.

5. Cocchetto DM, Sykes RS, Spector S. Paradoxical bronchospasm after use of inhalation aerosols: a review of the literature. J Asthma. 1991; 28:49–53.

6. Spooner LM, Olin JL. Paradoxical bronchoconstriction with albuterol administered by metered-dose inhaler and nebulizer solution. Ann Pharmacother. 2005; 39:1924–1927.

7. Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008; 31:143–178.

8. Shaw RJ, Anderson SD, Durham SR, Taylor KM, Schoeffel RE, Green W, Torzillo P, Kay AB. Mediators of hypersensitivity and "fog"-induced asthma. Allergy. 1985; 40:48–57.

9. Kashiwabara K, Itonaga K, Moroi T. Airborne water droplets in mist or fog may affect nocturnal attacks in asthmatic children. J Asthma. 2003; 40:405–411.
10. Skowronski ME, Ciufo R, Nelson JA, McFadden ER Jr. Effects of skin cooling on airway reactivity in asthma. Clin Sci Lond. 1998; 94:525–529.

11. Kaminsky DA, Bates JH, Irvin CG. Effects of cool, dry air stimulation on peripheral lung mechanics in asthma. Am J Respir Crit Care Med. 2000; 162:179–186.

12. Leddy JJ, Roberts A, Moalem J, Curry T, Lundgren CE. Effects of water immersion on pulmonary function in asthmatics. Undersea Hyperb Med. 2001; 28:75–82.
13. Chiba T, Yamauchi M, Nishida N, Kaneko T, Yoshizaki K, Yoshioka N. Risk factors of sudden death in the Japanese hot bath in the senior population. Forensic Sci Int. 2005; 149:151–158.

14. Hayes D Jr, Collins PB, Khosravi M, Lin RL, Lee LY. Bronchoconstriction triggered by breathing hot humid air in patients with asthma: role of cholinergic reflex. Am J Respir Crit Care Med. 2012; 185:1190–1196.
15. Aitken ML, Marini JJ. Effect of heat delivery and extraction on airway conductance in normal and in asthmatic subjects. Am Rev Respir Dis. 1985; 131:357–361.
16. Tranfa CM, Vatrella A, Parrella R, Bariffi F. Effect of ipratropium bromide and/or sodium cromoglycate pretreatment on water-induced bronchoconstriction in asthma. Eur Respir J. 1995; 8:600–604.
17. Keighley JF. Iatrogenic asthma associated with adrenergic aerosols. Ann Intern Med. 1966; 65:985–995.

18. Schöni MH, Kraemer R. Osmolality changes in nebulizer solutions. Eur Respir J. 1989; 2:887–892.
19. Rafferty P, Beasley R, Holgate ST. Comparison of the efficacy of preservative free ipratropium bromide and Atrovent nebuliser solution. Thorax. 1988; 43:446–450.

20. Wilkinson JR, Roberts JA, Bradding P, Holgate ST, Howarth PH. Paradoxical bronchoconstriction in asthmatic patients after salmeterol by metered dose inhaler. BMJ. 1992; 305:931–932.

21. Agostoni E, Gurtner G, Torri G, Rahn H. Respiratory mechanics during submersion and negative-pressure breathing. J Appl Physiol. 1966; 21:251–258.

22. Hong SK, Cerretelli P, Cruz JC, Rahn H. Mechanics of respiration during submersion in water. J Appl Physiol. 1969; 27:535–538.

23. Galdès-Sebaldt M, McLaughlin FJ, Levison H. Comparison of cold air, ultrasonic mist, and methacholine inhalations as tests of bronchial reactivity in normal and asthmatic children. J Pediatr. 1985; 107:526–530.

24. Kotaru C, Coreno A, Skowronski M, Ciufo R, McFadden ER Jr. Exhaled nitric oxide and thermally induced asthma. Am J Respir Crit Care Med. 2001; 163:383–388.

25. Chen WY, Horton DJ. Airways obstruction in asthmatics induced by body cooling. Scand J Respir Dis. 1978; 59:13–20.
26. Horton DJ, Chen WY. Effects of breathing warm humidified air on bronchoconstriction induced by body cooling and by inhalation of methacholine. Chest. 1979; 75:24–28.

27. Koskela HO, Koskela AK, Tukiaineu HO. Bronchoconstriction due to cold weather in COPD. The roles of direct airway effects and cutaneous reflex mechanisms. Chest. 1996; 110:632–636.
28. Gravelyn TR, Capper M, Eschenbacher WL. Effectiveness of a heat and moisture exchanger in preventing hyperpnoea induced bronchoconstriction in subjects with asthma. Thorax. 1987; 42:877–880.

29. Nieminen MM, Hill M, Irvin CG. Body temperature modulates the effect of platelet-activating factor PAF. on airways responsiveness in the rabbit. Agents Actions. 1991; 32:173–181.

30. Billen A, Dupont L. Exercise induced bronchoconstriction and sports. Postgrad Med J. 2008; 84:512–517.

31. Lazarinis N, Jorgensen L, Ekstrom T, Bjermer L, Dahlen B, Pullerits T, Hedlin G, Carlsen KH, Larsson K. Combination of budesonide/formoterol on demand improves asthma control by reducing exercise-induced bronchoconstriction. Thorax. 2014; 69:130–136.

32. Palmqvist M, Persson G, Lazer L, Rosenborg J, Larsson P, Lotvall J. Inhaled dry-powder formoterol and salmeterol in asthmatic patients: onset of action, duration of effect and potency. Eur Respir J. 1997; 10:2484–2489.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download