Abstract
Background
Objective
Methods
Results
Figures and Tables
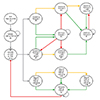 | Fig. 1Simplified presentation of the model structure. Arrow key: Red is flare; Green is response; Yellow is no response. AD, atopic dermatitis; ADCS, atopic dermatitis controlled state; CMF, standard cow's milk formula; PHF-W, partially hydrolyzed whey formula. Infant cohorts enter the model at birth [A] and initiate a 17-week course of PHF-W or CMF [B]. If and when AD develops [C], 3 treatment approaches were possible: (1) Formula change only [D]: The child enters ADCS on first-line treatment formula in case of response within 2 weeks [G], or, in case of nonresponse, she/he is switched to a second treatment formula [H]. Patients from the ADCS [G], who were previously treated with first-line treatment formula [D], upon experiencing a flare, are treated in 1 of 3 ways: adding first-line pharmacotherapy [E], switching to second-line treatment formula [H], or switching to second-line treatment formula + drug 1 [K]. In case of response to second-line of treatment formula [H], patients entered ADCS [I]. In case of nonresponse to second-line treatment formula [H] or a flare in ADCS [I], a first-line pharmacotherapy (drug 1) would be added [J]. For simplicity, the model assumes response is achieved at this point and the patient enters ADCS on AD treatment second-line treatment formula [I]. (2) Formula change combined with first-line pharmacotherapy (switch to first-line treatment formula + drug 1) [E]: Pharmacotherapy would end in case of response and the child would enter ADCS on first-line treatment formula [G]. Otherwise, they would switch to second-line treatment formula while remaining on the same pharmacotherapy [K]. At this point, response would occur and they enter ADCS on second-line treatment formula [I]. (3) First-line pharmacotherapy only (drug 1 along with the initial formula) [F]: The child experiences a response and enters ADCS on the original formula [L]. Otherwise they remain on the initial formula and switch to a second- and third-line pharmacotherapy (drug 2 [M] and drug 3 [N]) until response occurs, at which point the patient enters ADCS on the original formula [L]. Patients from the ADCS [L], who were previously treated within the addition of first-line pharmacotherapy only [F], upon experiencing flare, were assumed to be treated by either a change in formula, pharmacotherapy, or both. |
 | Fig. 2Scatter plot of 5,000 simulations from multivariate probabilistic sensitivity analysis. MYR, Malaysian Ringgit; QALY, quality adjusted life-year. |
Table 1
Epidemiologic inputs

uSA, univariate sensitivity analyses; PSA, probabilistic sensitivity analysis; AD, atopic dermatitis; CMF, standard cow's milk formula; RR, relative risk; PHF-W, partially-hydrolyzed whey-based formula.
*Due limited data sources, some value inputs were based on arbitrary variation in the univariate sensitivity analysis. †Source: von Berg et al. 2008 for PHF-W vs. CMF [10]. ‡Source: Expert panel. §The distribution of cases was varied simultaneously as scenario analysis of all severities in order to add up to 100%. Individual values are not relevant for univariate range thus not presented. ∥Source: Mortality data for children <5, specific to Malaysia (Source: World Bank data).
Table 2
Clinical management and effectiveness inputs*
uSA, univariate sensitivity analyses; PSA, probabilistic sensitivity analysis; AD, atopic dermatitis; PHF-W, partially-hydrolyzed whey-based formula; CMF, standard cow's milk formula.
*Source: Expert panel. †Due limited data sources, some value inputs were based on arbitrary variation in the univariate sensitivity analysis. ‡Instead of varying single proportion of case distribution, all 3 categories were varied simultaneously (formula switch, combined, and pharmacotherapy) using Dirichlet distributions. Individual values are not relevant for univariate range thus not presented.
Table 4
Summary of economic inputs (2013 MYR)*

MYR, Malaysian Ringgit; uSA, univariate sensitivity analyses; PHF-W, partially-hydrolyzed whey-based formula; CMF, standard cow's milk formula; EHF, extensively hydrolyzed formula; AD, atopic dermatitis.
*Distributions for costs in probabilistic sensitivity analysis were uniform. †Costs for PHF-W, CMF, Soy, and EHF were employed based on the availability and market share in the Malaysia using Packaged Food: Euromonitor from trade sources/national statistics. Recommended quantities from the package inserts were used to determine daily formula consumption quantity and varied based on age and percentage of feeding from breastfeeding. Complete daily formula quantity consumption available upon request. ‡Costs obtained from MIMS (http://www.mims.com/Malaysia/home/Index). Quantity applied varied based on AD severity and treatment line. §Costs are based on average fees charged in Malaysia. ∥Costs associated with time loss estimated using average hourly wages in Kuala Lumpur (MYR 51.3, http://www.salaryexplorer.com/salary-survey.php?loc=1515&loctype=3, last accessed March 30th 2014), labor force participation (64.40%) (Source: Nestle Malaysia affiliate), and hours spent was obtained from expert panel.
Table 5
Base-case model results for 6-year time horizon comparing PHF-W and CMF administration in children with a family atopic history

PHF-W, partially-hydrolyzed whey-based formula; CMF, standard cow's milk formula; MYR, Malaysian Ringgit; CI, confidence interval; US $, United States dollar; AD, atopic dermatitis; QALY, quality adjusted life-year.
*Includes only excess cost over and above the cost of CMF. †Percentile distributions (2.5th and 97.5th) to represent uncertainty around mean parameter value. ‡Dominance refers to a situation where one intervention (here, PHF-W) is said to dominate another (here, CMF) when its effectiveness is found to be higher and the costs lower.
ACKNOWLEDGEMENTS
Notes
CONFLICT OF INTEREST AJB, EGH, XJ, and MFB are and/or were employees and/or owner of Pharmerit International which received partial research funding from the Nestlé Nutrition Institute, Vevey, Switzerland to conduct this study. PD is an employee of Nestlé Research Center, Lausanne, Switzerland, which funded this study. AHAL, SW and PCK: None declared. The Nestlé Research Center, Lausanne, Switzerland, is part of Nestlé, which manufactures and commercializes a range of infant food products, including one of the products evaluated in this study. Pharmerit International retained independent control of the methodology and presentation of this study. The role of the 3 physicians in this study was to provide the clinical experience input and in this context does not directly or indirectly imply promotion of any infant formula products mentioned in this study.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download