Abstract
Oral platelet aggregation inhibitors are widely used for the treatment and prevention of cardiovascular diseases, including coronary stent thrombosis. Premature discontinuation following percutaneous coronary intervention would pose a grave risk of in-stent thrombosis, acute myocardial infarction and eventual death. Although they share the same mechanism of adenosine diphosphate P2Y12 platelet receptor inhibition, they belong to either the chemical class of thienopyridines (clopidogrel, prasugrel, and ticlopidine) or cyclopentyl-triazolo-pyrimidines (ticagrelor and cangrelor). This case describes the first documented cross-reactive hypersensitivity of clopidogrel towards both its fellow thienopyridine, prasugrel, as well as the structurally dissimilar ticagrelor, and its subsequent successful desensitisation.
Oral platelet aggregation inhibitors are widely used for the treatment and prevention of cardiovascular diseases, including coronary stent thrombosis. Premature discontinuation following percutaneous coronary intervention would pose a grave risk of in-stent thrombosis, acute myocardial infarction and eventual death. Although they share the same mechanism of adenosine diphosphate (ADP) P2Y12 platelet receptor inhibition, they belong to either the chemical class of thienopyridines (clopidogrel, prasugrel, and ticlopidine) or cyclopentyl-triazolo-pyrimidines (ticagrelor and cangrelor). This case describes the first documented cross-reactive hypersensitivity of clopidogrel towards both its fellow thienopyridine, prasugrel, as well as the structurally dissimilar ticagrelor, and its subsequent successful desensitisation.
A 57-year-old man with significant history of ischaemic heart disease underwent elective percutaneous coronary intervention to the proximal left anterior descending artery with insertion of two drug-eluting stents in February 2014. He was commenced on dual antiplatelet therapy with the addition of clopidogrel to his regular aspirin. His other past medical history included hypertension and hypercholesterolaemia. He had no previous history of drug allergy or atopy.
Eleven days after commencement of clopidogrel he developed a generalised urticarial rash including his scalp and palms, with associated peripheral paraesthesia. He was treated with antihistamines and his clopidogrel was changed to ticagrelor.
Thirty-six hours after the introduction of ticagrelor, he represented with facial angioedema associated with left sided chest pain and dysphagia without respiratory compromise. Following the cessation of ticagrelor, the symptoms resolved with intramuscular adrenaline and intravenous corticosteroids and he was discharged home after a period of observation.
Further cardiology consultation recommended the trial of prasugrel under close supervision. Within two hours, he developed an urticarial reaction involving the hands, feet and groin, which initially resolved with antihistamine. After the third dose of prasugrel, he experienced chest, left arm and abdominal discomfort, with periorbital and lip angioedema. Although haemodynamically stable, he was diaphoretic and required oxygen therapy. Again, he improved with intramuscular adrenaline, intravenous f luid and oral and intravenous antihistamines. All investigations were unremarkable including the tryptase level which was only collected 36 hours post symptom onset.
The idea of trialling a fourth antiplatelet agent, ticlopidine, was briefly considered. Given his reaction to three antiplatelet agents, and after reviewing the molecular structure of all four drugs (Fig. 1), as well as available literature on clopidogrel sensitisation and its cross-reactivity towards ticlopidine and prasugrel, there was significant concern about the risk of similar but more severe and rapid onset hypersensitivity reaction. The patient ultimately underwent clopidogrel desensitisation with prednisolone cover. He successfully completed the programme without complication and was maintained on strict daily dose afterwards. He continues to take clopidogrel without adverse effects four months post desensitisation.
Clopidogrel, an oral thienopyridine antiplatelet agent that inhibits the ADP-dependent pathway of platelet aggregation, is widely used in the prevention of vascular ischaemia associated with atherothrombotic events such as myocardial infarction and cerebrovascular accident. As percutaneous coronary stent insertions following acute coronary syndrome proliferated over the decade, the use of clopidogrel with aspirin in dual antiplatelet therapy has become commonplace. Due to its widespread use, hypersensitivity reactions to clopidogrel have also been increasingly recognised.
Clopidogrel is generally well tolerated but hypersensitivity reactions with pruritic rashes [1] occur in 6% of patients [2] while 1.5% requires drug discontinuation. The premature cessation of therapy increases the risk of atherothrombotic complications including death.
The conventional approach for persistent reaction has been to substitute an alternative thienopyridine such as ticlopidine [3]. However, ticlopidine is a less well tolerated drug giving toxic side effects of diarrhoea, neutropenia and thrombocytopenic purpura. There have also been reports of cross-reactivity between clopidogrel and ticlopidine [4], but not cross-reactivity between prasugrel [1] and ticagrelor [3].
In this case, ticagrelor, a nonthienopyridine with a similar structure to adenosine, was used as a substitute for clopidogrel. Despite structural dissimilarity, features of drug hypersensitivity with angioedema occurred within 72 hours of exposure. The angioedema suggests mast cell activation or bradykinin release but the time delay is contrary to that of an IgE-mediated immediate hypersensitivity mechanism. Challenge with prasugrel, a thienopyridine analogue of clopidogrel led to further urticarial reaction within two hours. The cross-reactive hypersensitivity between structurally dissimilar drugs with a similar mechanism of action further suggests this may not be an immunological mediated mechanism. We postulate the cross-reactivity between the structurally dissimilar clopidogrel and ticagrelor may be due to a common mechanism of action, such as P2Y12 platelet receptor inhibition. This is analogous to the hypersensitivity reactions seen in susceptible individuals towards structurally dissimilar aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) due to the common mechanism of cyclooxygenase-1 enzyme inhibition. However, this is speculative and requires further investigation.
Several clinical reports have demonstrated the efficacy of continued drug treatment uninterrupted using short-course corticosteroids and antihistamines [1, 2]. However, in the context of severe anaphylactic reactions, such as ours, the continuation of clopidogrel would not be clinically recommended. Clopidogrel desensitisation as a therapeutic alternative to ticlopidine has been described as safe and effective [5]. It can be performed in the outpatient setting to avoid premature discontinuation of therapy [6]. It has also been performed successfully with a short but intensive 3.5-hour regimen [7], compared to the standard 8-hour protocol [8].
The mechanisms of drug desensitisation and induction of tolerance are not fully understood. It is successful in both IgE (e.g., penicillin) and non-IgE (e.g., sulphonamide), as well as nonimmunologically mediated (e.g., aspirin and NSAIDs) drug hypersensitivity. However, upon successful desensitisation, a maintenance daily dose of the drug is required to preserve the desensitised state, irrespective of the underlying mechanism, as interruption of exposure will lead to redevelopment of the drug hypersensitivity. In our case, a published protocol [1, 6] was used (Table 1) and performed in a high dependency unit under close supervision with cardiac monitoring.
In conclusion, oral platelet aggregation inhibitors used in combination with aspirin to prevent in-stent thrombosis following percutaneous coronary intervention are not uncommonly associated with hypersensitivity reactions. Our case of cross-reactive hypersensitivity between thienopyridines (clopidogrel and prasugrel) and the structurally dissimilar cyclopentyl-triazolo-pyrimidine (ticagrelor) suggests the mechanism may be their common chemical action rather than immunological recognition. Nevertheless, desensitisation is a therapeutic option irrespective of the underlying mechanism.
Figures and Tables
References
1. Campbell KL, Cohn JR, Savage MP. Clopidogrel hypersensitivity: clinical challenges and options for management. Expert Rev Clin Pharmacol. 2010; 3:553–561.

2. Campbell KL, Cohn JR, Fischman DL, Walinsky P, Mallya R, Jaffrani W, Savage MP. Management of clopidogrel hypersensitivity without drug interruption. Am J Cardiol. 2011; 107:812–816.

3. Lokhandwala J, Best PJ, Henry Y, Berger PB. Allergic reactions to clopidogrel and cross-reactivity to other agents. Curr Allergy Asthma Rep. 2011; 11:52–57.

4. Makkar K, Wilensky RL, Julien MB, Herrmann HC, Spinler SA. Rash with both clopidogrel and ticlopidine in two patients following percutaneous coronary intervention with drug-eluting stents. Ann Pharmacother. 2006; 40:1204–1207.

5. Owen P, Garner J, Hergott L, Page RL 2nd. Clopidogrel desensitization: case report and review of published protocols. Pharmacotherapy. 2008; 28:259–270.

6. von Tiehl KF, Price MJ, Valencia R, Ludington KJ, Teirstein PS, Simon RA. Clopidogrel desensitization after drug-eluting stent placement. J Am Coll Cardiol. 2007; 50:2039–2043.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


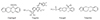

 XML Download
XML Download