Abstract
Serious tick-induced allergies comprise mammalian meat allergy following tick bites and tick anaphylaxis. Mammalian meat allergy is an emergent allergy, increasingly prevalent in tick-endemic areas of Australia and the United States, occurring worldwide where ticks are endemic. Sensitisation to galactose-α-1,3-galactose (α-Gal) has been shown to be the mechanism of allergic reaction in mammalian meat allergy following tick bite. Whilst other carbohydrate allergens have been identified, this allergen is unique amongst carbohydrate food allergens in provoking anaphylaxis. Treatment of mammalian meat anaphylaxis involves avoidance of mammalian meat and mammalian derived products in those who also react to gelatine and mammalian milks. Before initiating treatment with certain therapeutic agents (e.g., cetuximab, gelatine-containing substances), a careful assessment of the risk of anaphylaxis, including serological analysis for α-Gal specific-IgE, should be undertaken in any individual who works, lives, volunteers or recreates in a tick endemic area. Prevention of tick bites may ameliorate mammalian meat allergy. Tick anaphylaxis is rare in countries other than Australia. Tick anaphylaxis is secondarily preventable by prevention and appropriate management of tick bites. Analysis of tick removal techniques in tick anaphylaxis sufferers offers insights into primary prevention of both tick and mammalian meat anaphylaxis. Recognition of the association between mammalian meat allergy and tick bites has established a novel cause and effect relationship between an environmental exposure and subsequent development of a food allergy, directing us towards examining environmental exposures as provoking factors pivotal to the development of other food allergies and refocusing our attention upon causation of allergy in general.
Tick-induced allergies comprise large local reactions to tick bites, mammalian meat allergy or anaphylaxis following tick bites and tick anaphylaxis. Large local reactions to tick bites are the least severe manifestation of tick-induced allergies.
The more severe tick-induced allergies are mammalian meat anaphylaxis and allergy or anaphylaxis following tick bites and tick anaphylaxis.
The elegant demonstration of the mechanism of mammalian meat allergy as being due to sensitisation to the carbohydrate allergen, galactose-α-1,3-galactose (α-Gal), has generated considerable interest around the world, due to the unique nature of the allergen and the widespread distribution of sources of α-Gal in our lives. Moreover, the description of α-Gal specific-IgE has acted as a stimulus to the search for other hitherto unsuspected allergens involved in anaphylaxis.
The recognition of the association between mammalian meat allergy and tick bites, in establishing a novel cause and effect relationship between an environmental exposure and the subsequent development of a food allergy, has, however, provoked arguably even more intense interest around the world, as it directs us towards examining for environmental factors underlying the development of other food allergies and redirects our collective attention to potential factors pivotal to the causation of allergy in general.
Recently, the increasing prevalence of tick anaphylaxis in Australia has required a timely update of highly effective strategies for the secondary prevention of this condition. These tick bite avoidance and tick bite management strategies are likely to be successful in the primary prevention of both tick anaphylaxis and mammalian meat allergy following tick bites and tick anaphylaxis, provided they are disseminated widely enough to, and implemented by, those who live, work and/or enjoy their recreational pursuits in tick endemic regions around the world.
Large local reactions to tick bites (Fig. 1), due to their clinical similarities to venom-induced large local reactions (Table 1), in the majority of cases are likely to be late phase reactions mediated by tick specific-IgE [1].
Large local reactions to tick bites are most effectively treated by immobilisation of the affected area and elevation of the part above the heart (if possible) to aid resolution of the intense oedema, application of ice, administration of antihistamines daily with commencement as soon as possible after the tick bite and often administration of oral corticosteroids to suppress the inflammation, taken immediately when previous large local reactions to tick bites have occurred. Because these reactions are caused by a bite, that is, a puncture wound, and the tissues are grossly swollen, antibiotics are often given, particularly where the large local reaction has occurred in an area of the body where infection is more likely to occur e.g., around the buttocks and genitals or where infection would have more deleterious consequences e.g., around the eye.
Mammalian meat anaphylaxis following tick bite was first reported on 27 November 2007, in an abstract by van Nunen et al. [2] entitled "The Association between Ixodes holocyclus tick bite reactions and red meat allergy", published online in the Internal Medicine Journal in the proceedings of the eighteenth Annual Scientific Meeting of the Australasian Society of Clinical Immunology and Allergy (ASCIA), held in Fremantle, Australia earlier that month. The authors described 25 adult patients with positive skin prick tests (SPTs) and/or red meat specific-IgE detectable in their serum, 23 of whom had had allergic reactions following the ingestion of red meat (severe anaphylaxis after ingestion of red meat had occurred in 14/23). Twenty-four of 25 patients had a history of tick bite. The authors postulated an association between the history of prior tick bite and the development of red meat allergy. This work was later published in a slightly expanded form in the Medical Journal of Australia in May 2009 [3].
Again in 2007, O'Neil et al. [4] had reported a 22% incidence of grade 3 or 4 hypersensitivity reactions to cetuximab infusion in their patients in Tennessee and North Carolina when compared with an incidence of ≤3% nationally and internationally.
Following on from this observation, in March 2008, Chung et al. [5] published their work wherein they identified specific IgE directed against α-Gal as the cause of cetuximab-induced anaphylaxis. In this paper the authors referred to a series of patients (number unspecified) with IgE antibodies against α-Gal who reported having had episodes of anaphylaxis or severe angioedema 1 to 3 hours after eating beef or pork. They speculated that the environmental exposures which may have determined the regional variability seen in cetuximab anaphylaxis might be due to histoplasmosis, amoeba, ticks, coccidiomycosis, nematodes or cestodes [5]. Commins et al. [6] presented these data separately as an abstract at the American Academy of Allergy Asthma and Immunology (AAAAI) Meeting in March 2008, reporting 10 patients with recurrent anaphylaxis and angioedema triggered by exposure to beef and pork, all of whom possessed α-Gal specific-IgE. Fortuitously, in the same poster area, Dr Raymond Mullins, who had attended the 2007 ASCIA ASM, as the then President-elect of ASCIA, was presenting his work on the clinical significance of sensitisation to gelatine colloids in 800 patients, some of whom were cosensitised to mammalian meats [7].
In February 2009, Commins et al. [8] reported 24 patients with delayed anaphylaxis, angioedema or urticaria after consumption of red meat who possessed IgE specific for α-Gal. They noted "Interestingly, more than 80% of the patients in the present cohort report being bitten by ticks before having symptoms; a similar scenario has been recently described in a group of Australian patients" [8] and referenced the 2007 abstract by van Nunen et al. [2].
Since then, Platts-Mills, Commins and coworkers [5, 6, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19] together with our colleagues around the world [2, 3, 7, 10, 12, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37], have gathered extensive data and provided elegant proofs of the clinical observation by van Nunen et al. [2, 3], that tick bites are associated with the development of mammalian meat allergy.
Mammalian meat allergy is an emergent allergy, which has become increasingly prevalent in tick endemic areas of Australia and the south-eastern states of the United States (US).
The author has now personally seen, in all, well in excess of 600 individuals with mammalian meat allergy after tick bites (between 1985 and December 2014, with the great majority having presented from 2003 onwards) within a referral base of 440,000 people which includes the tick endemic areas nearby, and she currently diagnoses one-two new patients with this complaint each week [9]. Her colleagues in the same region have seen in excess of 200 other individuals with this condition [33]. Data in December 2013 indicated an estimated prevalence of 1/880 [37], however, data including 2014 diagnoses indicate 1/550 to be the current estimated prevalence. Cases have also been reported all along the eastern seaboard of the Australian continent and indeed, have been reported in patients from areas where ticks are nonendemic, due to recreational exposures to ticks in individuals resident in these nonendemic areas.
Particular "hotspots" along the coast remote from the Sydney basin, include the hinterland around Noosa in Queensland, Australia (personal communication from Drs Ted Chamberlain and Michael Tresillian) and the south coast of New South Wales, Australia (personal communication from Professor Paul Gatenby). The distribution of reported cases in Australia reflects the known distribution of the Australian paralysis tick, I. holocyclus (Fig. 2).
In the tick endemic areas in the Sydney basin, Australia, a diagnosis in adults of mammalian meat allergy, commonly anaphylaxis, appears to be as prevalent (estimate 0.12%, and higher when patients are included who have been diagnosed by other clinical immunologists in the same referral area) as the commonest food allergy in adults requiring adrenaline worldwide i.e., peanut allergy at 0.1% [38].
Platts-Mills and Commins [19] are aware of in excess of 1,000 individuals with mammalian meat allergy after tick bites (1/8,000) in Virginia alone (population over 8 million) and have estimated that in the south-eastern states of the US collectively in excess of 5,000 people have the complaint.
Cases of mammalian meat allergy after tick bite have now been reported from Australia, North America, Europe, Asia, Central America, and Africa. As ticks are widely distributed around the world it is not surprising that mammalian meat allergy after tick bites has been reported in several countries other than Australia and the US. The intriguing fact remains not that there have been so many countries in which this condition has been reported, but rather that the number of cases documented in these other countries has been so few.
In France in 2009, Jacquenet et al. [20] documented two cases of mammalian meat-induced anaphylaxis and confirmed by cetuximab skin testing that these patients were sensitised to α-Gal. Their group later presented an abstract at the 2012 AAAAI Meeting by Renaudin et al. [21] describing 6 α-Gal positive patients with delayed urticaria and angioedema due to mammalian meat allergy. Fourteen patients were described from France in 2012, all allergic to pork or beef kidney, all of whom had positive skin tests to cetuximab and had α-Gal specific-IgE detectable in their serum [22]. Information regarding exposure to ticks was not included in these series. Morisset et al. [23] at the European Academy of Allergy and Clinical Immunology-World Allergy Organization (EAACI-WAO) Meeting in Milan in 2013, described an additional single case in whom yoghurt allergy and ricotta cheese anaphylaxis developed after a repeat tick bite in a patient with previously established mammalian meat anaphylaxis which had been confirmed by detection of α-Gal specific-IgE in the serum.
Nunez et al. [24] in 2011 reported 5 patients from Spain with delayed mammalian meat-induced anaphylaxis. All patients had positive beef and cetuximab skin tests, all had beef, lamb and pork specific-IgE demonstrable and all but one reported previous tick bites. The authors noted that the predominant tick species in the area of Spain where their patients lived is Ixodes ricinus [24].
In their case report of delayed anaphylaxis following ingestion of gelatine-containing sweets in a patient sensitised to alpha-gal, Caponetto et al. [25] noted that they cared for a total of 21 patients with red meat anaphylaxis.
In addition, Commins and Platts-Mills [9] have commented that Jappe [26] is said to have identified patients with mammalian meat allergy and cetuximab and α-Gal specific-IgE via serological studies and referenced her review of the topic. Since then, Jappe [34, 35] and Jappe et al. [36] have summarised the findings in 32 German patients with mammalian meat allergy following tick bites, recruited by her and her colleagues from all over Germany. The presence of α-Gal sensitisation and sensitisation to other epitopes was noted and they correlated these findings with the clinical features exhibited by their patients.
In late 2013, in Switzerland, Michel et al. [27] published online their study of 2 patients with mammalian meat allergy, noting that SPTs and intradermal tests with cetuximab were positive in both, as were basophil activation tests.
Hamsten et al. [28] reported initially 5 patients with mammalian meat-induced anaphylaxis who had presumed exposure to I. ricinus which is common in the greater Stockholm area. All 5 patients possessed α-Gal specific-IgE [28]. This series was later expanded and they have now described 39 patients in all with mammalian meat allergy and α-Gal specific-IgE [10].
A male patient aged 67 with delayed pork and beef anaphylaxis and delayed urticaria after ingesting lamb was described by Lee et al. [29] in 2012. The diagnosis was confirmed by intradermal cetuximab skin testing [29]. My Korean colleague, Professor Yoon-Seok Chang has personally communicated (Copenhagen, EAACI, June 2014) that several more cases have occurred in Korea.
In Japan in 2012, Sekiya et al. [30] reported a single case, a woman aged 74, who after a tick bite developed mammalian meat and cow's milk anaphylaxis confirmed by an oral challenge with pork.
Two people who have lived all of their lives in a farming community near the coast in the Republic of South Africa have contacted the author regarding their long-standing mammalian meat allergies after tick bites which appeared in adulthood and one of these patients has gelatine allergy as well. A third person, from Zimbabwe, has recounted his experience with the condition to the author.
One person from Costa Rica in Central America has also informed the author of her mammalian meat allergy which she believes has followed tick bites.
As far as the author is aware, no cases have yet been reported from South America, however, Ixodidae ticks are known to be present and 3 species frequently parasitise humans: Arvicanthis neumanni in 46 known localities in Argentina, Amblyomma triste in 21 known sites in Uruguay and Amblyomma parvum in 27 known areas in Argentina-Brazil, with human infestation by Ixodes spp. species virtually unknown in South America with only a single report from the entire continent [39].
The most reasonable explanation for the increasing prevalence of mammalian meat allergy in both Australia and the US is an increase in host numbers (bandicoots and other small native mammals flourishing in Australia [37] and the increase in the white tailed deer population in south-eastern US [13]).
Mammalian meat allergy is exceedingly rare in adults in the absence of a prior tick bite. The clinical features of mammalian meat allergy are summarised in Table 2.
Kennedy et al. [14] identified 45 children from Virginia, US, who had both a clinical history consistent with mammalian meat-induced delayed anaphylaxis or recurrent urticaria and IgE antibody specific for α-Gal. All patients had a history of tick bite prior to α-Gal detection and 39 of the 45 children had experienced persistent reactions to tick bites. This finding of local reactivity is in keeping with the fact that 24/25 patients in van Nunen's study [2, 3] had large local reactions at the site of their tick bites and Caponetto et al. [25] noting persistent reactions at the bite site. Absorption studies in three sera determined that the cow's milk specific-IgE detected was due entirely to the presence of α-Gal in the cow's milk and these findings led Kennedy et al. [14] to recommend α-Gal testing and a search for mammalian meat allergy in those aged 5 and over living in tick endemic areas and with a new diagnosis of cow's milk allergy. The authors concluded mammalian meat allergy in children is not uncommon and noted that it mirrors their experience in adults [25].
The clinical features of mammalian meat allergy are now well defined and it is known to affect both adults and children. A minority of mammalian meat allergy sufferers will also react to the α-Gal in mammalian gelatine, mammalian milks and other mammalian meat derivatives e.g., bovine colostrum.
Whilst avoidance of mammalian meat per se can be accomplished reasonably easily, those who have clinical sensitivity to gelatine have benefited from the work by Mullins et al. [12] showing the presence of α-Gal in gelatine and bovine products. Their findings underpin our advice to patients regarding the risks of reacting to gelatine, in particular, as this can be administered intravenously in therapeutic preparations e.g., gelatine-containing colloids, a route of administration which increases the possibility of anaphylaxis [12]. Mullins et al. [12] also noted gelatine allergy may be the initial presentation of mammalian meat allergy, recorded clinical reactivity in mammalian meat allergy to both intravenous and oral gelatine, reported a small number of patients with positive gelatine tests and negative mammalian meat tests who reacted to gelatine challenge and who remained free of anaphylaxis avoiding both mammalian meat and gelatine and noted again an historical association between tick bite exposure, sensitization and allergy to red meat. The patients reported, from Canberra (and across to the Pacific coast), Australian Capital Territory, Australia, were exposed to I. holocyclus [12].
Confirmation of a diagnosis of an allergic reaction due to mammalian meat involves serological testing for α-Gal specific-IgE and mammalian meat specific-IgE [8, 24, 27, 31] or cetuximab skin testing [20, 24, 29, 30] or skin prick testing by prick-prick tests with fresh, raw, organic meat or extracts thereof [3, 8, 21, 24]. SPT reactions to mammalian meats are characteristically small. Their significance may be missed by both the patient and the physician if they are unfamiliar with this fact [8, 14]. Jappe et al. [36] have found that their Cetuximab-Blot technique was more sensitive in detecting specific-IgE in patients with urticaria alone, whilst the α-Gal ImmunoCAP was more useful in confirming the more severe reactors.
The cornerstone in the management of mammalian meat allergy is avoidance of mammalian meat. A dietitian familiar with the pitfalls experienced by those living with mammalian meat allergy is the ideal person to guide a newly diagnosed person with mammalian meat allergy [41], maintain iron stores in those with a longstanding requirement for avoiding mammalian meat and ensure dietary adequacy of vitamin B12 when a mammalian meat free diet needs to be prescribed [41]. When sensitisation to gelatine and cow's milk products is present, help from a specialized dietitian is invaluable for the patient as they will be able to keep such individuals safe by indicating the cryptic sources of gelatine in our diet [7, 12], instructing them in label reading and researching alternative food choices.
Avoidance of mammalian meat in the diet is of proven benefit in those with anaphylaxis after ingestion of mammalian meat [8, 12, 14, 31]. In those with a stable pattern of delayed urticaria alone it may be possible for them to continue eating some mammalian meat or some preparations of mammalian meats, provided they reduce the amounts they consume, be consistent with cooking methods and avoid cofactors when ingesting mammalian meat. Exclusion is usually practised, however, when angioedema is the clinical manifestation of mammalian meat allergy, as patients are more intolerant of episodes of angioedema due to the limitation of function which often occurs and the patient's perception of angioedema as being unsightly. Gut symptoms can be severe and in this situation dietary exclusion is often preferred by the patient.
SPT (prick-prick) can be useful in liberalizing the diet safely e.g., negative prick-prick tests to bacon, ham and prosciutto usually indicate that these foods will be tolerated and hard cheeses can be tolerated by many sufferers, whereas soft cheeses may not be tolerated and SPT will usually predict whether or not this is the case. α-Gal is reportedly not detectable after boiling cow's milk and heat treatment of cow's milk in general, as seen with many other food allergens, can render some forms of allergen tolerable e.g., cow's milk taken in tea or coffee can almost always be safely consumed.
The safety of mammalian meat allergic patients is improved when they understand the role of cofactors in determining whether or not they will suffer an anaphylaxis on any given occasion after ingestion of mammalian meat. It is useful, especially when a severe anaphylaxis has occurred, to state to the patient that they may not react on every occasion that they consume mammalian meat, i.e., that they have an "anytime risk, but not an every time risk" of having an allergic reaction to mammalian meat.
A convalescent tryptase level should be obtained in all who have suffered a severe anaphylaxis after ingestion of mammalian meat to exclude coexisting mastocytosis [32].
Occasional patients report having desensitised themselves. Such information needs to be viewed with caution, as it may merely represent a loss of sensitivity in that individual.
Desensitisation has been disappointing in the few milder cases where it has been trialled in our patients in uncontrolled circumstances e.g., in patients with gut symptoms alone (the author, personal communication). In general, the severity of the mammalian meat reactions in the majority of patients coupled with the delayed nature of the reactions make desensitisation a less attractive option than it is for many other IgE mediated food allergies.
Before initiating treatment with certain therapeutic agents (e.g., cetuximab, gelatine-containing substances, bovine artificial blood), a careful assessment of the risk of anaphylaxis, including serological analysis for α-Gal specific-IgE, should be undertaken in any individual who works, lives, volunteers or recreates in a tick endemic area, particularly where a history is obtained of a tick bite prior, or of mammalian meat or gelatine allergy.
Both physicians and pharmacists need to inform mammalian meat allergic patients of the risks inherent in taking cetuximab [5, 15] as fatal reactions have occurred with its use [15]. The risk of fatal anaphylaxis is well documented and well known to allergic diseases physicians, however, this risk still needs to be known to patients before they receive therapy with cetuximab. Sources of gelatine in therapeutic agents should be flagged e.g., in vaccines, capsules, tablets and suppositories and in collagen-containing agents (including implants) [12]. Physicians, pharmacists and health supplement purveyors need to be aware of the implications of any mammalian meat-derived content in proprietary products e.g., bovine colostrum.
The widespread distribution of α-Gal in agents other than foods in our environment has created difficulties in keeping patients safe from allergic reactions. Just as α-Gal is important in cancer therapy, it likewise plays a role in xenotransplantation. Aloha-Gal, as would be expected, has been identified and recently quantified in porcine heart valve bio-prostheses [42]. Allergic reactions have been described where α-Gal sensitisation has appeared to play a role in adverse events following cardiac valve xenografts. In 2011, Fournier et al. [43] concluded that their single patient, who succumbed to afebrile blood culture negative endocarditis after receiving four porcine heart valve bio-prostheses, did so as a result of pork allergy. Testing for the presence of α-Gal specific-IgE was not performed in their patient and beef specific-IgE was absent. More recently, Mozzicato et al. [44] described adverse events perioperatively and postoperatively in two of three α-Gal positive patients who received porcine bio-prostheses. Fortunately, symptoms in the two affected patients settled quickly with treatment. The risk of such reactions, however, needs to be added to a burgeoning list of possible agents which might cause allergic reactions in mammalian meat allergic patients. Presumably, residual α-Gal, which is known to be present on all bio-prosthetic valves (except those which have been decellularised), is either washed off the valve into the circulation following placement of the valve or reacts with α-Gal specific-IgE whilst still adhering to the valve, despite measures to counter its presence such as treatment with glutaraldehyde. Regulatory authorities need to be cognizant of mammalian meat allergy in formulating disclosure rules for medicinal preparations and medical devices.
As tick bites are known to be the provoking factor in the development of mammalian meat allergy, and given that many allergies tend to remit with the passage of time, mammalian meat allergy would be expected to lessen over time in the absence of further tick bites. There is limited evidence for this currently [14]. The chance of remission seems greater for mammalian meat allergy contracted from bites from Amblyomma americanum (the Lone Star tick present in the south-eastern states of the US) than in patients sensitised by bites from I. holocyclus (the Australia paralysis tick). Likewise the time taken to achieve a remission (1-2 years in those bitten by A. americanum) versus few reports of remission in less than a decade in those bitten by those sensitised by bites from I. holocyclus (the author, personal communication).
Possibly as long as 35 million years ago, selective evolutionary pressure to suppress the expression of alpha-galactosyltransferase began to be exerted in higher primates, most likely in response to an infectious agent or agents to which higher mammals were exposed [45]. By 28 million years ago, as suggested by the comparative studies in primates by Galili et al. [45, 46, 47], α-Gal was no longer elaborated virtually in higher primates-Man, the apes and Old World monkeys, and anti-α-galactosyl IgG (anti-Gal) (IgG directed against α-Gal) is produced. Alpha-Gal is produced in large amounts in nonprimate mammals (>106 epitopes per cell) [47]. Detection of anti-Gal binding sites in some strains of Escherichia coli, Klebsiella, and Salmonella by Galili et al. [46, 47], suggests α-Gal is present in the bacterial polysaccharides in the outer membranes of the bacterial flora in human intestines and that the large amounts of anti-Gal produced in the human (constituting approximately 1% of circulating IgG in human serum) is due to the continued stimulation of B lymphocytes producing anti-Gal in response to these enterobacteria [46, 47]. Support for this hypothesis is forthcoming from the work of Posekany et al. [48], who demonstrated the production of cytolytic anti-gal antibodies in alpha-galactosyltransferase knockout mice following their inoculation with E. coli.
Clearly, loss of tolerance to α-Gal cannot be complete, as mammalian meat is consumed by many humans regularly. This tolerance has been viewed as being analogous to the tolerance induced in oral immunotherapy. We also tolerate the few hundred cryptic α-Gal epitopes which appear on our erythrocytes during the red cell ageing process [47]. Anti-Gal contributes to the removal of these senescent erythrocytes by binding to these α-Gal epitopes and thereby opsonizing these cells for phagocytosis [47].
In 1981, Aalberse et al. [49] noted the marked cross-reactivity of some patients' sera against multiple plant allergens and insect venoms. Later work from Kurosaka et al. [50] and Tretter et al. [51], established the structural basis for this observation as being due to protein-linked carbohydrate, an asparagine-linked N-glycan containing the cross-reactive carbohydrate core α1,3-fucose in the case of horseradish peroxidase [50] and in bee venom [51], eliciting production of antibodies across all classes as noted by van Die and Cummings [52]. In general, glycan-specific IgE antibodies do not elicit allergic reactions and their significance overall in eliciting allergic reactions per se has been largely dismissed by van der Veen et al. [53].
Alpha-Gal is a carbohydrate moiety, an oligosaccharide or glycan, constituted from two galactose molecules by the enzyme, alpha-galactosyl-transferase. Alpha-gal is a most unusual allergen in that it has a greater propensity to provoke anaphylaxis than any other known cross-reactive carbohydrate determinant.
The other serious tick-induced allergy is tick anaphylaxis. Anaphylaxis to hard ticks was initially reported in 1940 [54], again in the 1960s [55]. Prevalent in tick-endemic areas in Australia [56-61], it is very rarely reported in other countries [62, 63, 64]. Worldwide Ixodes spp. (I. holocyclus [56, 57, 58, 59, 60, 61], I. pacificus [62], and I. ricinus [63, 64]) are causative mainly. Tick anaphylaxis is secondarily preventable by prevention and appropriate management of tick bites [61, 62, 65]. Analysis of tick removal techniques in tick anaphylaxis sufferers offers insights into primary prevention of both tick and mammalian meat anaphylaxis [61]. The features of tick anaphylaxis are summarised in Table 3 and its typical prevalence is indicated in Fig. 3.
The available data indicate that prevention of a recurrence of tick anaphylaxis is achievable by killing the tick in situ [61, 62, 65].
Behaviour changes prevent subsequent tick bites in the majority of sufferers [61]. Tick bite prevention measures [41, 66] and regular inspections for ticks, particularly at bedtime, are worthwhile. The success of measures designed to prevent tick anaphylaxis recurrence is simply assessed by the lack of reaction after their use. Practicability of such measures is paramount. Killing the tick in situ is the aim. Successful methods include the use of ether-containing agents to freeze the tick where it is embedded [41, 61]. This method has the advantage of being easy to use and the sprays are readily available. Once the tick is dead it should be left to drop off the host. If this is not feasible, it should be removed without compressing the salivary glands which would, unfortunately, inject allergen into the host's vasculature [61, 65]. The use of fine-tipped forceps coupled with gentle upwards traction is often advised for tick removal [67]. This approach is aimed at limiting transmission of infection and is not suitable for preventing allergic reactions to ticks.
Using fine-tipped forceps requires a great deal of skill and good eyesight. The at-risk group, mature tick anaphylaxis sufferers, volunteer that they are unable to remove a tick thus. The use of fine-tipped forceps should therefore be restricted to health professionals in an appropriate facility and the use of tweezers discouraged in the population at large.
Unfortunately, people almost invariably translate advice to use fine-tipped forceps to using household tweezers, which compress the tick and its feeding chamber within the host's skin and thereby squeeze allergen into the host's vascular bed. The tick must not be disturbed, scratched or pulled out as this is well recognised to result in an immediate anaphylaxis in those sensitised [59, 61, 65].
In 1988, Gauci et al. [57] published their findings regarding their detection by radio-immunoassay of IgE specific for the Australian paralysis tick, I. holocyclus in the sera of individuals allergic to these ticks. Characterisation of the allergens by radioimmunoassay and Western blot analysis identified the allergens as being predominantly in the salivary glands of ticks [58]. They found two common allergens, of approximately 28 and 35 kDa each [58] and noted several minor allergens of 45, 50, and 55 kDa [58]. The two major allergens were found to be present only in adult females and nymphs and not in larval ticks.
Over the next several years, tick allergens were further investigated by Broady and coworkers [68, 69, 70]. Broady [68] summarised these findings at the TiARA (Tick-induced Allergies Research and Awareness) Meeting on 20 August 2013. From 1992-1998, Dorey [69] in work for which a PhD was awarded, identified a wider range of tick allergens using immunoblotting, demonstrated that allergen presence varied depending upon the stage of engorgement of the tick, noted cross-reactivity between tick and dust mite allergens and identified tropomyosin as one of these cross-reactive allergens. In 2008 and 2009, Padula [70] identified galactosylated tick protein allergens in the sera of tick anaphylaxis sufferers and showed that these were homologous with previously reported arthropod allergens from a number of sources both insect- and crustacean-derived. Following on from this work, Broady noted that Singh in 2011, in a BSc Honours thesis, showed several galactosylated proteins were present in tick extract and demonstrated that 40% of the IgE bound in the sera of their two patients with tick anaphylaxis was directed against galactosylated moieties whilst the remaining 60% reacted to other protein allergens. The tick allergens shown to contain galactose are paramyosin, arginine kinase, troponin C, and fructose-biphophate aldolase, all of which are known arthropod and crustacean allergens. Paramyosin is a known allergen in dust mites, arginine kinase in dust mites, cockroach, moth, and shrimp. Broady noted after his detailed analysis of the structure of these proteins that only the tick arginine kinase has a protein sequence which predicts glycan attachment (O-glycosylation at T155) and only tick troponin C has a predicted glycosylation site (N-glycosylation at N104) [68].
Restating the work of Galili [45, 46, 47], if an epigenetic influence was brought to bear upon the higher primates, that is, if the production of anti-Gal was evolutionarily advantageous in the context of exposure to an infectious agent or agents many, many years ago, then for those higher primates capable of inactivating the alpha-galactosyltransferase gene, there would exist a selective pressure towards their survival. The resultant loss of tolerance for α-Gal would then set the scene for the development of an allergy to α-Gal in otherwise susceptible individuals.
This allergy to α-Gal develops only in those who are predisposed. Alpha-Gal allergy appears to be expressed more severely when relatively large amounts of α-Gal enter the blood stream over a short period. The temporal dependency of mammalian meat allergy following tick bites has been documented ex vivo by Commins et al. [18]. The allergen is likely transferred via the lymphatics to drain back into the central circulation (personal communication Aalberse RC, via Platts-Mills TA, London, EAACI June, 2010). The recent case reports by Mozzicato et al. [44] of short-lived perioperative or postoperative allergic reactions in α-Gal positive patients following their receipt of their bovine or porcine valve bio-prostheses are also in keeping with this mechanism and its temporal dependency, as the reactions seen mimicked the anaphylactic reactions seen in mammalian meat allergy.
Production of IgE specific for α-Gal requires the action of a promoting factor (the tick) generating 'allergy proneness', that is, Th2 predominance in the host, due to the action of the tick upon the host's immune system. In keeping with this skewing of the host's immune system towards a Th2 cytokine profile is the demonstration by Ferreira and Silva [71] of the selective promotion of such a profile in the mouse following successive tick infestations with Rhipicephalus sangueineus.
The delivery by the tick of the allergen by injection, the most effective route for provoking an allergy, takes full advantage of the milieu of allergy proneness which has been established. A switch from IgG antibody production in the host to production of IgE specific for either or both α-Gal or tick salivary proteins ensues, depending upon the individual susceptibility to develop allergy to these moieties. Rispens et al. [16] distinguished two types of immune response to α-Gall epitopes: a "typical" IgG2 response, presumed due to response to gut bacteria, and an "atypical", Th2-like response leading to production of IgG1 and IgE in addition to IgG2. They concluded that their results suggest that IgE to a carbohydrate antigen can be formed (probably as part of a glycoprotein or glycolipid) even against a background of bacterial immune stimulation with essentially the same antigen.
The role of basophil activation may be crucial to the occurrence of more severe reactions to either or both of these allergens [18]. In the case of mammalian meat anaphylaxis, by entry of α-Gal directly into the circulation following absorption and circulation in the lymphatics, and in cases of tick anaphylaxis, by direct injection into the host bloodstream following disturbance of the tick when it has not been killed in situ and left to drop off, or when compression of salivary glands and/or the tick feeding chamber, or both, have occurred as a result of inappropriate tick removal techniques being employed.
Tick-induced allergies are emergent allergies. Mammalian meat allergy is becoming increasingly prevalent in tick-endemic areas of Australia and the US and it is being reported world-wide. Tick anaphylaxis, which occurs virtually only in Australia, is also becoming increasingly prevalent.
The nature of the allergen, α-Gal, explains the unusual clinical features seen in patients with mammalian meat allergy following tick bite. Injection of the allergen into the host's skin aids in the development of α-Gal allergy, whilst its mode of digestion is responsible for the temporal profile of the reactions and their severity, by virtue of their entry directly in to the host's circulation.
The environmental exposure, to tick bites, promotes the development of a Th2 cytokine profile in the host and by virtue of the ability of tick proteins to be galactosylated, when a class switch is generated in the host to production of IgE specific for a tick protein, α-Gal specific-IgE is also elaborated, which then results in this cross-reactive carbohydrate moiety being recognized in mammalian meat ingested by the host, engendering an allergic reaction in the host, in those individuals susceptible to developing this allergy. Simultaneously, tick bites expose the host to glycosylated tick salivary proteins and in those individuals susceptible to tick anaphylaxis, this generates a more typical IgE mediated allergy, an immediate anaphylaxis to a protein foreign to the host.
Tick bites, the stimulus to the development of tick-induced allergies, both mammalian meat allergy following tick bites and tick anaphylaxis, are avoidable, given that appropriate advice is disseminated within, and followed by, the population at large. Further, in the event of a lapse in implementing effective tick avoidance measures, prevention of the transmission of significant quantities of allergen is achievable by using appropriate tick removal techniques.
Tick-induced allergies offer a simple and elegant paradigm for the development of noninhalant allergies. These conditions may be viewed as "allergy in a box" i.e., "Allergy-how to get it, how to lose it and how to not get it in the first place!".
Tick-induced allergies may therefore be the first allergies where we not only understand how they developed a priori, but also the first allergies which we may be able to largely prevent, both primarily and secondarily.
Figures and Tables
 | Fig. 1Confluent large local reactions to 2 tick bites (Ixodes holocyclus). Adapted with permission of Stephen L. Doggett, Senior Hospital Scientist, Pathology West, ICPMR (level 3), Westmead Hospital, Locked Bag 9001, Westmead NSW 2145, Australia. |
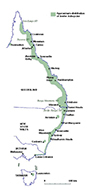 | Fig. 2Distribution of Ixodes holocyclus (Australian paralysis tick). Map adapted from Roberts FHS (1970) Australian Ticks. Yeerongpilly, QLD, Australia by TAGS Inc., Bill Conroy & Norbert Fischer. |
 | Fig. 3Numbers of patients with anaphylaxis to insects presenting to a single allergy consultant practice in Sydney, Australia 2011-2013. Adapted with permission of Stephen L. Doggett, Senior Hospital Scientist, Pathology West, ICPMR (level 3), Westmead Hospital, Locked Bag 9001, Westmead NSW 2145, Australia. Illustrations of bee and wasps courtesy of WikiCommons. |
References
1. Graft DF, Schuberth KC, Kagey-Sobotka A, Kwiterovich KA, Niv Y, Lichtenstein LM, Valentine MD. A prospective study of the natural history of large local reactions after Hymenoptera stings in children. J Pediatr. 1984; 104:664–668.

2. van Nunen SA, Fernando SL, Clarke LR, Boyle RX. The association between Ixodes holocyclus tick bite reactions and red meat allergy [abstract]. Intern Med J. 2007; 37:Suppl 5. A132.
3. van Nunen SA, O'Connor KS, Clarke LR, Boyle RX, Fernando SL. An association between tick bite reactions and red meat allergy in humans. Med J Aust. 2009; 190:510–511.

4. O'Neil BH, Allen R, Spigel DR, Stinchcombe TE, Moore DT, Berlin JD, Goldberg RM. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007; 25:3644–3648.
5. Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D, Slebos RJ, Zhou Q, Gold D, Hatley T, Hicklin DJ, Platts-Mills TA. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008; 358:1109–1117.
6. Commins S, Lucas J, Hosen J, Satinover SM, Borish L, Platts-Mills TA. Anaphylaxis and IgE antibodies to galactose-alpha-1,3-galactose (alphaGal): insight from the identification of novel IgE ab to carbohydrates on mammalian proteins [abstract]. J Allergy Clin Immunol. 2008; 121:S25.
7. Mullins RJ. Clinical significance of sensitisation to gelatine colloids in 800 patients [abstract]. J Allergy Clin Immunol. 2008; 121:S26.
8. Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, Woodfolk JA, Platts-Mills TA. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009; 123:426–433.
9. Commins SP, Platts-Mills TA. Tick bites and red meat allergy. Curr Opin Allergy Clin Immunol. 2013; 13:354–359.

10. Hamsten C, Tran TA, Starkhammar M, Brauner A, Commins SP, Platts-Mills TA, van Hage M. Red meat allergy in Sweden: association with tick sensitization and B-negative blood groups. J Allergy Clin Immunol. 2013; 132:1431–1434.

11. Wickner PG, Commins S. The first central american cases of delayed meat allergy with galactose-alpha-1,3-galactose positivity clustered among field biologists in panama [abstract #736]. In : 2014 AAAAI Annual Meeting; 2014 February 28-March 4; San Diego, CA, USA. Milwaukee, WI: American Academy of Allergy, Asthma & Immunology;2014.
12. Mullins RJ, James H, Platts-Mills TA, Commins S. Relationship between red meat allergy and sensitization to gelatin and galactose-α-1,3-galactose. J Allergy Clin Immunol. 2012; 129:1334–1342.e1.

13. James HR, Commins SP, Kelly LA, Pochan SL, Workman LJ, Mullins RJ, Platts-Mills TA. Further evidence for tick bites as a cause of the IgE responses to alphagal that underlie a major increase in delayed anaphylaxis to meat [abstract]. J Allergy Clin Immunol. 2011; 127:2 Suppl. AB243.
14. Kennedy JL, Stallings AP, Platts-Mills TA, Oliveira WM, Workman L, James HR, Tripathi A, Lane CJ, Matos L, Heymann PW, Commins SP. Galactose-α-1,3-galactose and delayed anaphylaxis, angioedema, and urticaria in children. Pediatrics. 2013; 131:e1545–e1552.

15. Pointreau Y, Commins SP, Calais G, Watier H, Platts-Mills TA. Fatal infusion reactions to cetuximab: role of immunoglobulin e-mediated anaphylaxis. J Clin Oncol. 2012; 30:334. author reply 335.

16. Rispens T, Derksen NI, Commins SP, Platts-Mills TA, Aalberse RC. IgE production to α-gal is accompanied by elevated levels of specific IgG1 antibodies and low amounts of IgE to blood group B. PLoS One. 2013; 8:e55566.

17. Schroeder N, Eccles J, Klaffky EJ, Platts-Mills TAE. Cellular infiltrate induced by bites from the tick Ambylomma americanum in subjects with or without IgE to galactose-alpha-1,3-galactose (alpha-gal) [abstract #783]. In : 2014 AAAAI Annual Meeting; 2014 February 28-March 4; San Diego, CA, USA. Milwaukee, WI: American Academy of Allergy, Asthma & Immunology;2014.
18. Commins SP, James HR, Stevens W, Pochan SL, Land MH, King C, Mozzicato S, Platts-Mills TA. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2014; 134:108–115.
19. Platts-Mills TA, Commins SP. Emerging antigens involved in allergic responses. Curr Opin Immunol. 2013; 25:769–774.

20. Jacquenet S, Moneret-Vautrin DA, Bihain BE. Mammalian meatinduced anaphylaxis: clinical relevance of anti-galactose-alpha-1,3-galactose IgE confirmed by means of skin tests to cetuximab. J Allergy Clin Immunol. 2009; 124:603–605.
21. Renaudin J, Jacquenet S, Metz-Favre C, Baudoin E, Engel F, de Blay F, Moneret-Vautrin DA. Interest of specific IgE Measurement for galactose-alpha-1,3-galactose in unexplained urticaria and angioedema, predominantly nocturnal: about 6 cases [abstract]. J Allergy Clin Immunol. 2012; 129:AB177.
22. Morisset M, Richard C, Astier C, Jacquenet S, Croizier A, Beaudouin E, Cordebar V, Morel-Codreanu F, Petit N, Moneret-Vautrin DA, Kanny G. Anaphylaxis to pork kidney is related to IgE antibodies specific for galactose-alpha-1,3-galactose. Allergy. 2012; 67:699–704.

23. Morisset M, Richard C, Zanna H, Jacquenet S, Morel-Codreanu F, Jarlot S, Hosotte M, Kanny G. Allergy to cow's milk related to IgE antibodies specific for galactose-alpha-1,3-galactose [abstract #1617]. In : European Academy of Allergy and Clinical Immunology-World Allergy Organization World Allergy & Asthma Congress; 2013 June 22-26; Milan, Italy. Milwaukee, WI: World Allergy Organisation;2013.
24. Nunez R, Carballada F, Gonzalez-Quintela A, Gomez-Rial J, Boquete M, Vidal C. Delayed mammalian meat-induced anaphylaxis due to galactose-α-1,3-galactose in 5 European patients. J Allergy Clin Immunol. 2011; 128:1122–1124.e1.
25. Caponetto P, Fischer J, Biedermann T. Gelatin-containing sweets can elicit anaphylaxis in a patient with sensitization to galactose-α-1,3-galactose. J Allergy Clin Immunol Pract. 2013; 1:302–303.

26. Jappe U. Update on meat allergy. α-Gal: a new epitope, a new entity? Hautarzt. 2012; 63:299–306.
27. Michel S, Scherer K, Heijnen IA, Bircher AJ. Skin prick test and basophil reactivity to cetuximab in patients with IgE to alpha-gal and allergy to red meat. Allergy. 2014; 69:403–405.

28. Hamsten C, Starkhammar M, Tran TA, Johansson M, Bengtsson U, Ahlen G, Sallberg M, Gronlund H, van Hage M. Identification of galactose-α-1,3-galactose in the gastrointestinal tract of the tick Ixodes ricinus; possible relationship with red meat allergy. Allergy. 2013; 68:549–552.
29. Lee JH, Kim JH, Kim TH, Kim SC. Delayed mammalian meat-induced anaphylaxis confirmed by skin test to cetuximab. J Dermatol. 2013; 40:577–578.

30. Sekiya K, Fukutomi Y, Nakazawa T, Taniguchi M, Akiyama K. Delayed anaphylactic reaction to mammalian meat. J Investig Allergol Clin Immunol. 2012; 22:446–447.
31. van Nunen SA, Mastroianni M, Fulton R, Fernando S, Basten A. Clinical utility of allergen specific-IgE measurement in the diagnosis of mammalian meat/s-induced anaphylaxis [abstract]. Intern Med J. 2011; 41:Suppl 4. 4–22.
32. van Nunen SA, Zaininger A, Clarke LR, Coyle L, Stevenson W, Fernando SL. Severe anaphylaxis provoked by IgE-mediated reactions to food (red meat) in two patients with systemic mastocytosis [abstract]. Intern Med J. 2009; Suppl 5. A145.
33. Baumgart KW, Mok A, Sivertsen T, Mullins R, van Nunen S. Allergen-specific IgE after anaphylaxis to tick or mammalian/marsupial meat in Australia [abstract]. In: European Academy of Allergy and Clinical Immunology Congress; 2014 June 7-11; Copenhagen, Denmark. Allergy. 2014; 69(S99):1–646.
34. Jappe U. Ein Disacchand-Epitop als Ausloser fur verschiedene Medikamentenallergen und eine Nahrungsmittelallergie auf Fleisch.Bedeulung fur die Aufdarung von Pathomechanismen und Sensibilesen ungswegen. Allergologie. 2013; 36:413–414.
35. Jappe U. Anaphylaxis caused by hidden food allergens: the α-Gal syndrome. Allergologie. 2014; 37:S265–S274.
36. Jappe U, Platts-Mills T, Przybilla B, Kreft B, Ludwig A, Walker A, Varga R, Fischer J, Biedermann T, Rueff F, Merk H, Wagner N, Treudler R, Worm M, Wulff S, Saloga J, Kromminga A, Becker WM, Petersen A. IgE-reactivity to galactose-[alpha]-1,3-galactose: a prospective multicenter study on meat allergy, urticaria and anaphylaxis [abstract #202]. In: Thirty-first European Academy of Allergy and Clinical Immunology Congress; 2012 June 16-20; Geneva, Switzerland. Allergy. 2012; 67:Suppl 96. 96.
37. van Nunen S. Galactose-alpha-1,3-galactose, mammalian meat and anaphylaxis: a world-wide phenomenon? Curr Treat Options Allergy. 2014; 1:262–277.

38. Ben-Shoshan M, Harrington DW, Soller L, Fragapane J, Joseph L, St Pierre Y, Godefroy SB, Elliott SJ, Clarke AE. A population-based study on peanut, tree nut, fish, shellfish, and sesame allergy prevalence in Canada. J Allergy Clin Immunol. 2010; 125:1327–1335.

39. Guglielmone AA, Beati L, Barros-Battesti DM, Labruna MB, Nava S, Venzal JM, Mangold AJ, Szabó MP, Martins JR, Gonzalez-Acuna D, Estrada-Pena A. Ticks (Ixodidae) on humans in South America. Exp Appl Acarol. 2006; 40:83–100.

40. van Nunen SA, Said MG, Batty G. Use of expanded search criteria in diagnosing mammalian meat allergy provoked by previous tick bites in individuals who do not live where ticks are endemic [abstract #2602]. In : European Academy of Allergy and Clinical Immunology-World Allergy Organization World Allergy & Asthma Congress; Milan, Italy; Milwaukee, WI: World Allergy Organisation;2013.
41. TiARA (Tick-induced Allergies Research and Awareness). Royal North Shore Hospital, Sydney, Australia: Allergies Provoked by Ticks [Internet]. 2013 Mar 26. Available from: http://www.tiara.org.au/.
42. Naso F, Gandaglia A, Bottio T, Tarzia V, Nottle MB, d'Apice AJ, Cowan PJ, Cozzi E, Galli C, Lagutina I, Lazzari G, Iop L, Spina M, Gerosa G. First quantification of alpha-Gal epitope in current glutaraldehyde-fixed heart valve bioprostheses. Xenotransplantation. 2013; 20:252–261.

43. Fournier PE, Thuny F, Grisoli D, Lepidi H, Vitte J, Casalta JP, Weiller PJ, Habib G, Raoult D. A deadly aversion to pork. Lancet. 2011; 377:1542.

44. Mozzicato SM, Tripathi A, Posthumus JB, Platts-Mills TA, Commins SP. Porcine or bovine valve replacement in 3 patients with IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol Pract. 2014; 2:637–638.

45. Galili U, Clark MR, Shohet SB, Buehler J, Macher BA. Evolutionary relationship between the natural anti-Gal antibody and the Gal alpha 1: 3Gal epitope in primates. Proc Natl Acad Sci U S A. 1987; 84:1369–1373.
46. Galili U, Mandrell RE, Hamadeh RM, Shohet SB, Griffiss JM. Interaction between human natural anti-alpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun. 1988; 56:1730–1737.

47. Galili U. Evolution and pathophysiology of the human natural anti-alpha-galactosyl IgG (anti-Gal) antibody. Springer Semin Immunopathol. 1993; 15:155–171.

48. Posekany KJ, Pittman HK, Bradfield JF, Haisch CE, Verbanac KM. Induction of cytolytic anti-Gal antibodies in alpha-1,3-galactosyltransferase gene knockout mice by oral inoculation with Escherichia coli O86:B7 bacteria. Infect Immun. 2002; 70:6215–6222.
49. Aalberse RC, Koshte V, Clemens JG. Immunoglobulin E antibodies that crossreact with vegetable foods, pollen, and Hymenoptera venom. J Allergy Clin Immunol. 1981; 68:356–364.

50. Kurosaka A, Yano A, Itoh N, Kuroda Y, Nakagawa T, Kawasaki T. The structure of a neural specific carbohydrate epitope of horseradish peroxidase recognized by anti-horseradish peroxidase antiserum. J Biol Chem. 1991; 266:4168–4172.

51. Tretter V, Altmann F, Kubelka V, Marz L, Becker WM. Fucose alpha 1,3-linked to the core region of glycoprotein N-glycans creates an important epitope for IgE from honeybee venom allergic individuals. Int Arch Allergy Immunol. 1993; 102:259–266.
52. van Die I, Cummings RD. Glycans modulate immune responses in helminth infections and allergy. Chem Immunol Allergy. 2006; 90:91–112.
53. van der Veen MJ, van Ree R, Aalberse RC, Akkerdaas J, Koppelman SJ, Jansen HM, van der Zee JS. Poor biologic activity of cross-reactive IgE directed to carbohydrate determinants of glycoproteins. J Allergy Clin Immunol. 1997; 100:327–334.

54. McKay WJ. Tick bite and allergy. Med J Aust. 1940; 1:458–459.
57. Gauci M, Stone BF, Thong YH. Detection in allergic individuals of IgE specific for the Australian paralysis tick, Ixodes holocyclus. Int Arch Allergy Appl Immunol. 1988; 85:190–193.
58. Gauci M, Stone BF, Thong YH. Isolation and immunological characterisation of allergens from salivary glands of the Australian paralysis tick Ixodes holocyclus. Int Arch Allergy Appl Immunol. 1988; 87:208–212.
59. Gauci M, Loh RK, Stone BF, Thong YH. Allergic reactions to the Australian paralysis tick, Ixodes holocyclus: diagnostic evaluation by skin test and radioimmunoassay. Clin Exp Allergy. 1989; 19:279–283.

60. Rappo TB, Cottee AM, Ratchford AM, Burns BJ. Tick bite anaphylaxis: incidence and management in an Australian emergency department. Emerg Med Australas. 2013; 25:297–301.

61. van Nunen S, Basten A, Cowdery N, Anderson D, Canfield P, Broady K, Said M, Ratchford A, Doggett S, Arns E, Cook G, Ginsborg S, Mullins R. Tick removal techniques practised by tick anaphylaxis sufferers. Australasian Society of Clinical Immunology and Allergy (ASCIA) Conference, Melbourne. Intern Med J. 2014; 44:Suppl 4. 1–37.
62. Van Wye JE, Hsu YP, Lane RS, Terr AI, Moss RB. IgE antibodies in tick bite-induced anaphylaxis. J Allergy Clin Immunol. 1991; 88:968–970.

63. Moneret-Vautrin DA, Beaudouin E, Kanny G, Guerin L, Roche JF. Anaphylactic shock caused by ticks (Ixodes ricinus). J Allergy Clin Immunol. 1998; 101(1 Pt 1):144–145.
64. Fernandez-Soto P, Davila I, Laffond E, Lorente F, Encinas-Grandes A, Perez-Sanchez R. Tick-bite-induced anaphylaxis in Spain. Ann Trop Med Parasitol. 2001; 95:97–103.
65. Stone BF. Dealing with ticks affecting humans in Australia, especially the Australian paralysis tick Ixodes holocyclus. St. Lucia (AU): Department of Microbiology & Parasitology, School of Molecular & Microbial Sciences, University of Queensland;2000.
66. Australasian Society of Clinical Immunology and Allergy (ASCIA). Tick allergy [Internet]. Balgowlah (AU): Australasian Society of Clinical Immunology and Allergy;2014. 2014 Dec 2. Available from: http://www.allergy.org.au/patients/insect-allergy-bites-and-stings/tickallergy.
67. Due C, Fox W, Medlock JM, Pietzsch M, Logan JG. Tick bite prevention and tick removal. BMJ. 2013; 347:f7123.

68. Broady KW. Presentation of findings: tick allergens. In : TiARA (Tickinduced Allergies Research and Awareness) Committee Meeting; 2013 August 20; Sydney: Royal North Shore Hospital;2013.
69. Dorey C. Allergens of the Australian Paralysis Tick, Ixodes holocyclus [dissertation]. Sydney: University of Technology, Sydney;1998.
70. Padula MP. The development of proteomic techniques to study the Australian Paralysis Tick, Ixodes holocyclus [dissertation]. Suydney: University of Technology, Sydney;2008.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download