Abstract
Bee venom injection therapy is an alternative treatment sometimes used for chronic inflammatory diseases, including rheumatoid arthritis and multiple sclerosis, to reduce pain. Several chemical components of bee venom have anti-inflammatory effects, and apitoxin, one of the mixed components, has been used for pain prevention therapy. However, there have been no large-scale investigations regarding the efficacy or side effects or apitoxin. In this study, a case of serum sickness reaction that developed after receiving bee venom injection therapy is reported.
Bee venom therapy is a therapeutic method that has been used for several millennia. Before the syringe was invented, this method involved a therapist stinging the patient directly with a bee. Today however, it is used as a complementary and alternative medicine for controlling chronic pain, but usually only by traditional medical therapists in certain parts of the world. Bee venom has been used to control pain and inflammation in patients with multiple sclerosis, rheumatoid or osteoarthritis, and other chronic inflammatory diseases [12],
Apitoxin is a complex composition of various chemicals and proteins including melittin, adolapin, apamin, mast cell degranulating peptide (MCD), histamine, dopamine, and some enzymes such as phospholipase A2 (PLA2) and hyaluronidase [3]. Of those components, melittin and adolapin are responsible for the anti-inflammatory efficacy [45].
Despite some evidence that apitoxin is effective, there are many concerns that injecting bee venom as a treatment is unwise given the lack of understanding of its chemical components, mechanism of action, and potential component interactions. In addition, the exact dosing, toxicity, and incidence of side effects have not been established. The side effects of bee venom injection therapy may be similar to those of bee venom immunotherapy, but the number of studies performed is insufficient to determine this accurately. In addition, an accurate methodology has not been established, and no large-scale clinical trials have been performed. Some trials have suggested that bee venom injection is effective, but these were based on only a small number of subjects [1].
In this study, a case of serum sickness that developed after receiving bee venom injection therapy is discussed.
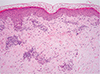
A 31-year-old woman visited with fever, arthralgia on the knees and ankles, generalized myalgia, a rash, and swelling on her lower legs and trunk. The patient experienced a mild herniated intervertebral disc in her lumbar spine with lower back pain for 5 months before admission. The patient had been treated with bee venom injection therapy on multiple occasions for 4 months by a traditional therapist, and the pain had improved without any side effects. Back pain had recurred 4 weeks prior, and the patient had again received an injection of bee venom. On each occasion, 1-mL apitoxin was injected directly into the patient's lower back muscles, and two to four injections were given each time. Four days after re-treatment, reddish skin lesions and swelling developed on the patient's legs and then spread to the trunk (Fig. 1), with pain in the knees and ankles as well as abdominal discomfort. At the time of transfer, purpuric erythema of multiple sizes (from pea to walnut) occurred, along with itching and a stinging sensation (Fig. 1). The patient's body temperature was 37.9℃, and other vital signs were stable. Laboratory tests revealed a white blood cell count of 29,700/µL (neutrophil, 70.8%; eosinophil, 1.0%), hemoglobin levels of 11.9 g/dL, a platelet count of 102,000/µL, C-reactive protein levels of 12.4 mg/dL, and an erythrocyte sedimentation rate of 22 mm/hr. Mild elevation of liver enzymes (aspartate aminotransferase, 58 IU/L; alanine aminotransferase, 44 IU/L) and decreased levels of C3 (24 mg/dL; normal range, 88-206 mg/dL) and C4 (16 mg/dL; normal range, 13-75 mg/dL) were noted. The C1q immune complex was negative. Serum electrolytes and kidney function were normal. Antinuclear antibody, antidouble stranded DNA antibodies and antineutrophil cytoplasmic antibody were negative. The patient tested negative for honeybee-, white faced hornet-, yellow jacket-, and paperwasp-specific IgE, but tested positive for honeybee venom-specific IgG1 (4.5 µg/dL, 2+). A chest x-ray did not reveal any abnormalities. A skin biopsy revealed urticarial vasculitis (Fig. 2). The patient was treated with a systemic corticosteroid (prednisolone 30 mg) and an antihistamine and recovered after 10 days.
Serum sickness, a complement-mediated reaction, usually occurs 4-10 days after the introduction of foreign proteins and is characterized by a fever, skin rash, lymphadenopathy, arthralgia, and myalgia. The frequency and severity of serum sickness are associated with the dose of the exposed protein and the specificity of the allergen [6].
In this case, fever, skin rash, abdominal discomfort, and abnormal hepatic function occurred 4 days after injection of apitoxin. The fact that the patient tested positive for honeybee-specific IgG and had decreased levels of complement C3, along with the other accompanying symptoms, suggests activation of a complex-mediated type III immune reaction. However the patient tested negative for four kinds of bee-specific (honeybee, white faced hornet, yellow jacket, and paper wasp) IgE, and thus the specific cause of the type I immune reaction cannot be determined in this patient.
An increase in serum IgE and an alteration in IgG do not always occur in patients with a bee sting-induced serum sickness reaction [6]. The altered antibody level after repeated antigen injection has not been well investigated; however, the large amount of antigen exposure probably results in the stimulation of IgG antibody production, which is required for the development of an immune complex reaction.
Some components of bee venom have definite anti-inflammatory effects. Melittin is a major component that makes up 50% of all bee venom components and inhibits the enzymatic activity of PLA2, which leads to the release arachidonic acid [4]. However, the injection of melittin provokes edema and local inflammation in a rheumatoid arthritis animal model [4]. Melittin mediates immune-modulating effects by blocking activation of the transcription factors nuclear factor-kappaB and STAT3 and by regulating mitochondrial apoptosis-related genes [78]. Melittin is also thought to inhibit proliferation of rheumatoid synovial cellsby inducing apoptosis through CASP3 activation [9] and induces apoptosis in apoptosis-resistant synoviocytes in rheumatoid arthritis [8]. Adolapin is another candidate that elicits an anti-inflammatory effect, but it accounts for 2% of bee venom [5]. In addition, there is less pharmacologic evidence for the action of adolapin compared with mellitin. Adolapin has been shown to induce an antinociceptive effect by increasing the pain threshold [5] and an anti-inflammatory effect by inhibiting cyclooxygenase activity inhibition and ultimately prostaglandin synthesis [10].
Despite these studies, these molecules have only been evaluated in terms of their anti-inflammatory effects, but their interaction with other bee venom components has not been determined. In addition, no large-scale clinical trials have been performed to determine the side effects of bee venom injection therapy. The most common side effects are allergic reactions, naturally, but even the actual incidence of allergic reactions has not been known until now. Recently, a fatal case of disseminated intravascular coagulation that developed after bee venom injection therapy was reported [11], but there may be many unreported cases of apitoxin injection side effects. Of the clinical studies performed to determine the effectiveness of bee venom injection therapy, none were randomized control trials, the total sample sizes were too small to confirm the effectiveness of this approach [1], and some overestimated the effectiveness [1]. Essentially, apitoxin may be the same as allergens used in bee venom immunotherapy. Apitoxin was extracted from the venom of Apis mellifera by an electric shock method, but in contrast to bee venom immunotherapy, the effectiveness of apitoxin injection therapy has not been demonstrated, and no dosage recommendations or side effects have been established. Even patients receiving venom immunotherapy are instructed to administer emergency epinephrine in some countries.
These chemicals found in bee venom have not been standardized, and the adverse effects of each chemical are not completely understood. Bee venom injection therapy has not been evaluated using modern medical or pharmacological methods, and no rigorous clinical studies have been performed. To ensure safer practice of bee venom injection, more studies are needed.
Figures and Tables
References
1. Lee MS, Pittler MH, Shin BC, Kong JC, Ernst E. Bee venom acupuncture formusculoskeletal pain: a review. J Pain. 2008; 9:289–297.
3. Billingham ME, Morley J, Hanson JM, Shipolini RA, Vernon CA. Letter: Ananti-inflammatory peptide from bee venom. Nature. 1973; 245:163–164.
4. Hartman DA, Tomchek LA, Lugay JR, Lewin AC, Chau TT, Carlson RP. Comparison ofantiinflammatory and antiallergic drugs in the melittin- and D49 PLA2-inducedmouse paw edema models. Agents Actions. 1991; 34:84–88.
5. Koburova KL, Michailova SG, Shkenderov SV. Further investigation on theantiinflammatory properties of adolapin: bee venom polypeptide. Acta Physiol Pharmacol Bulg. 1985; 11:50–55.
6. Reisman RE, Livingston A. Late-onset allergic reactions, including serumsickness, after insect stings. J Allergy Clin Immunol. 1989; 84:331–337.
7. Stuhlmeier KM. Apis mellifera venom and melittin block neither NF-kappaB-p50-DNA interactions nor the activation of NF-kappa B, instead they activatethe transcription of proinflammatory genes and the release of reactive oxygenintermediates. J Immunol. 2007; 179:655–664.
8. Kim SK, Park KY, Yoon WC, Park SH, Park KK, Yoo DH, Choe JY. Melittin enhancesapoptosis through suppression of IL-6/sIL-6R complex-induced NF-κB and STAT3activation and Bcl-2 expression for human fibroblast-like synoviocytes inrheumatoid arthritis. Joint Bone Spine. 2011; 78:471–477.
9. Hong SJ, Rim GS, Yang HI, Yin CS, Koh HG, Jang MH, Kim CJ, Choe BK, Chung JH. Bee venom induces apoptosis through caspase-3 activation in synovial fibroblasts of patients with rheumatoid arthritis. Toxicon. 2005; 46:39–45.

10. Shkenderov S, Koburova K. Adolapin: a newly isolated analgetic andanti-inflammatory polypeptide from bee venom. Toxicon. 1982; 20:317–321.
11. Jung JW, Jeon EJ, Kim JW, Choi JC, Shin JW, Kim JY, Park IW, Choi BW. A fatalcase of intravascular coagulation after bee sting acupuncture. Allergy Asthma Immunol Res. 2012; 4:107–109.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download