Abstract
Background
Children with a diagnosis of cross-reactive hypersensitivity to both paracetamol and nonsteroidal anti-inflammatory drugs are limited in their choice of antipyretics.
Objective
The aim of this pilot study is to evaluate the feasibility of using a Chinese proprietary medicine, Yin Qiao San (YQS), for fever relief.
Methods
A single centre, open label, prospective clinical trial exploring the tolerability and feasibility of using YQS for fever relief in children who are unable to use conventional antipyretic medications. Children between 1-18 years of age with hypersensitivity to multiple antipyretics were recruited. Eligible participants underwent an oral provocation test with YQS. Children who passed the oral provocation test were instructed to take a prescribed dose of YQS when the temperature was >38.0℃ and continued till the fever settled. Time taken for fever resolution and any adverse events were collected.
Results
A total of 21 children, mean age 10.7 years, had a diagnosis of paracetamol and ibuprofen hypersensitivity. All except one patient successfully tolerated an oral challenge of YQS. Of the 88 doses of YQS taken for fever over 38.0℃, 16 (18%) had documented temperature reduction 2 hours after ingestion and 30 (34%) had documented temperature reduction 4 hours after ingestion. There were 2 reports of urticaria after YQS use which were attributed to flare of recurrent spontaneous urticaria during the illness. None of the patients developed symptoms of circulatory compromise or respiratory distress.
Both acetaminophen and ibuprofen are widely used among children as analgesic and antipyretic agents. As a group, nonsteroidal anti-inflammatory drugs (NSAIDs) antagonize inflammation by blocking cyclooxygenase enzymes known as COX-1 and COX-2. While acetaminophen has no significant action on peripheral COX-1 and COX-2, it causes selective inhibition of the enzyme COX-3 which is found in the brain and spinal cord [1]. Thus although having almost no anti-inflammatory effects and strictly speaking not an NSAID medication, acetaminophen is an inhibitor of prostaglandin synthesis.
NSAIDs are frequently involved in hypersensitivity drug reactions [23] and account for 24.6% of adverse drug reactions among Singaporean children [4]. The majority of reactions are not caused by immunological mechanisms but by a combination of the pharmacological inhibition of prostaglandin synthesis via the COX pathways and an uncompensated increase in leukotriene production from arachidonic acid. This may explain the published incidence of sensitivity to acetaminophen among patients with cross-reactive NSAID hypersensitivity, on average around 4-25% [567].
Young children with a diagnosis of hypersensitivity to both acetaminophen and NSAIDs are limited in their choice of antipyretics. We were informed by some parents that they had used herbal preparations with no adverse problems.
Many of these remedies have "classical" formulations in the official Chinese pharmacopaea and are marketed as ready-made mixtures, also named Chinese proprietary medicines. Yin Qiao San (YQS) is one such proprietary formulation, a combination of 10 herbs which reduce heat and treat fever (Table 1).
No formal studies evaluating YQS as a treatment for fever in children have been published so far. Data however suggests that some of the ingredients have valid antipyretic and anti-inflammatory effects under laboratory conditions [8910111213141516]. Other herbal preparations containing similar herbal ingredients have been shown to be effective and safe in the treatment of acute bronchiolitis [17], acute bronchitis [18] and influenza [19].
This pilot study aims to evaluate the feasibility of using YQS for fever relief in children with documented hypersensitivity to acetaminophen and ibuprofen.
A single centre, open label, prospective clinical trial exploring the tolerability and feasibility of using Yin Qiao San for the relief of fever and acute febrile illness symptoms in children unable to use conventional antipyretic medications. The work was reviewed and approved by the Singhealth Centralised Institutional Review Board. The study was supported by a traditional medicine grant provided by Singhealth Foundation.
Patients were recruited from the paediatric allergy clinic at KK Women's and Children's Hospital (KKH) in Singapore. Between January 2010 and June 2011, patients with a diagnosis of hypersensitivity to multiple antipyretics were evaluated by an experienced paediatric allergist.
Patients were included if they were between 1-18 years of age, had a diagnosis of multiple antipyretic hypersensitivity and were otherwise well except for atopy associated problems such as atopic dermatitis, allergic rhinitis, asthma and food allergy. We excluded from the analysis patients who were <1 year of age, had a prior history of seizures or chronic disease except those listed above and from whom we could not obtain consent.
Eligible participants underwent an oral provocation test with YQS in the outpatient clinic. Those who had an adverse reaction during the initial oral provocation test or during the study course were withdrawn from the study. Data on the challenge episode were entered as an event in the analysis of YQS usage safety.
The doses were determined during discussions with a traditional Chinese medicine (TCM) physician at Eu Yan Sang, who acted as an external advisor for our study. The dosing regimen is detailed in Table 2.
Participants who passed the oral provocation test with YQS were instructed to use the prescribed dose of YQS every 8 hourly when they had an upper respiratory tract infection (fever, runny nose, cough, and sore throat) and the temperature was >38℃. They would continue to take YQS until the fever settled and each episode of illness was recorded in a diary card.
Where the diagnosis could not be convincingly made on history alone, hypersensitivity to NSAIDs and paracetamol was confirmed with an oral provocation test performed according to our previous published protocol [20].
In brief, all oral provocation tests were performed in the outpatient clinic. Before test administration, patients were examined and vital signs including heart rate, respiratory rate, blood pressure, oxygen saturation and peak flow measurements were recorded. Challenge was not performed if the patient received any orally administered medications at the time. Administration of all antihistamines was stopped 1 week before testing. Patients were administered increasing doses of the suspected NSAID or paracetamol at intervals of 60 minutes up to a total of three administrations in 1 day. For oral provocation tests with YQS, patients were administered a single dose of YQS as per the dosing regimen in Table 2. Patients were monitored in the clinic for at least 2 hours after the last ingested dose. If cutaneous and/or respiratory symptoms or alterations in vital signs appeared, the procedure was stopped and the symptoms evaluated and treated. If no symptoms appeared during the oral provocation test, the therapeutic dose was deemed to have been administered successfully.
All tests were performed in the respiratory laboratory by an experienced technician using commercial allergen extracts and the GreerPick skin prick test device (Greer Laboratories, Lenoir, NC, USA). A wheal diameter of 3 mm or more in excess of the negative control was considered a positive test result. The skin prick test panel included: house dust mite mix (Dermatophagoides farina 5,000 AU/mL + Dermatophagoides pteronyssinus 5,000 AU/mL, standardised); Blomia tropicalis 0.2-mg protein/mL, 50% vol/vol glycerol; cockroach mixture (Periplaneta americana, Blattella germanica); mould mix (Alternaria alternate, Aspergillus fumigatus, Candida albicans, Cladosporium herbarum, Curvularia lunata, Penicillium expansum); cat hair (Felis catus [domesticus] 10,000 (bioequivalent allergy unit/mL, standardised); dog epithelia (Canis familiaris).
Temperature monitoring was standardised and an ear thermoscan thermometer was provided for each study patient. Instructions were given to measure the core temperature at least four times a day when they had an upper respiratory tract infection and to start treatment once temperature was >38.0℃. After taking medication, they had to measure their core temperature hourly until the temperature was <37.5℃.
Participants were followed up for 1 year with 4 scheduled visits throughout the study course. Subjects who passed the oral provocation test were enrolled during the first visit and given diary cards, information leaflets and sachets of YQS.
Subsequent visits were scheduled every 3 months, during which the diary card would be reviewed and compliance to study drugs was reinforced. Monthly telephone consults were conducted between visits to check on use of the study drug and any adverse reactions.
A post study survey was conducted to evaluate the tolerability and satisfaction score with the use of YQS.
Between January 1, 2010 and June 30, 2011, 21 patients, including 6 male patients (29%), 14 Chinese patients (67%) and 5 Malay patients (24%), with a mean age of 10.7 years (range, 2.1-16.2 years) were recruited. Allergic disease was associated in 90% of patients; 86% had allergic rhinitis, 24% had allergic conjunctivitis, and 24% had asthma. Recurrent urticaria and/or angioedema were present in 57% of the patients while 24% had a positive family history of hypersensitivity to at least one NSAID. Skin prick tests were positive for >1 aeroallergen in 71% of patients. The clinical and demographic data are detailed in Table 3.
All 21 patients had a clinical or drug provocation test proven diagnosis of paracetamol and ibuprofen cross-reactive hypersensitivity (Table 4). In addition, 1 patient failed an etoricoxib provocation test.
All except one patient successfully tolerated an outpatient oral challenge of YQS. This patient was 16 years old at the time of enrollment. She had allergic rhinitis, allergic conjunctivitis as well as recurrent angioedema. During the oral provocation test with 3 g of YQS, the patient developed periorbital urticaria and angioedema. There was no evidence of circulatory compromise or respiratory distress. She did not require additional medication or hospitalization.
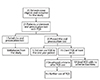
Of 20 patients who tolerated the oral challenge of YQS, 15 patients used YQS at least once during a fever episode (Fig. 1). The remaining 5 patients did not experience fever during the study period. There were 2 reports of urticaria after YQS use which were attributed to flare of recurrent spontaneous urticaria during the illness. Both patients had a history of recurrent episodes of unprovoked urticaria and/or angioedema, allergic rhinitis and skin prick tests that were positive to house dust mites and cockroach. The first patient developed periorbital swelling 8 hours after the first dose of YQS. The rash lasted 24 hours and further treatment was not required. The second patient developed periorbital swelling 30 minutes after the first dose of YQS. This patient was treated with antihistamines and the rash resolved 3 days later. Both patients did not receive further doses of YQS. None of our patients developed hypotension or symptoms of circulatory compromise.
There were 30 febrile episodes in the one year period and the mean duration of fever was 2.0 days (range, 1-5 days). A mean of 2 doses per fever episode (range, 1-5 doses) was used for fever reduction. Of the 88 doses of YQS taken for fever over 38℃, 16 (18%) had documented temperature reduction 2 hours after ingestion and 30 (34%) had documented temperature reduction 4 hours after ingestion. In a post study survey, 13 of 15 patients who used YQS indicated that YQS was effective in reducing fever and that they would use it in the future. This included the 2 patients who experienced urticaria after YQS use. Of the remaining 2 patients, 1 gave an equivocal response and 1 was lost to follow up. All 13 patients who responded positively indicated that YQS was a safe drug and gave a mean score of 7.9 (range, 6-10) on a satisfaction scale of 1 to 10, with 1 being unsatisfactory and 10, most satisfactory.
TCM is based on the concept of Yin and Yang with the disease state due to Yin-Yang imbalance. TCM works by promoting the harmonization of Yin and Yang. Many remedies have been documented in the Chinese pharmacopaea for hundreds of years. They have not been subjected to the rigor of a randomized controlled trial but have been used by generations of Chinese as trusted remedies.
One of the major concerns with using TCM is the batch to batch variability of herbal products that affect the eventual content of these formulations. In Singapore, the manufacture and distribution of Chinese proprietary medicine is regulated by the Health Sciences Authority of Singapore, requiring all manufacturers and companies to conform to Good Manufacturing Practice in accordance with the Pharmaceutical Inspection Convention/Cooperation Scheme.
TCM is an alternative medical option or even first line medicine for many Singaporeans [21]. In a questionnaire survey conducted in KKH, about two-thirds of the children seen at the specialist outpatient clinics received some form of herbal therapy. Most self medicate without a formal consultation. It is interesting to note that events which would normally have caused great concerns if "Western medicine " was prescribed were viewed very differently if the treatment was herbal therapy.
Children with early onset NSAID hypersensitivity appear to have a significantly increased likelihood of reacting to acetaminophen [22]. In particular, Asian children, especially atopic children, seem to be at an increased risk for hypersensitivity reactions to COX-inhibitors [20]. In adults, drugs that selectively inhibit the COX-2 enzyme are safe alternatives in the majority of patients with cross-intolerant NSAID hypersensitivity [2324]. There are no COX-2 inhibitors licensed for use among children.
Here we have described a series of 21 children with confirmed cross-reactive NSAID and acetaminophen hypersensitivity who underwent a drug provocation test with YQS. All except one patient tolerated the oral challenge and there were no severe adverse reactions in those who used YQS for fever relief.
The most common associated allergic diseases were allergic rhinitis (86%), allergic conjunctivitis (24%) and asthma (24%). Sensitisation to >1 aeroallergens was present in a large number of the patients. This association of NSAID hypersensitivity with atopy and asthma is consistent with previous publications [2526].
YQS was found to be potentially effective in reducing fever during an upper respiratory tract infection. Although quick fever reduction was only documented in 18% of the patients, 34% documented fever relief 4 hours after ingestion and the majority (87%) responded positively in the post study survey. This included the 2 patients who experienced a flare of recurrent spontaneous urticaria during the illness. Even the presence of a rash that would normally have caused great concern would not deter these patients from using the herbal preparation in the future. Over and above the fever reduction, reduction in discomfort associated with the febrile illness could explain the positive response to YQS in the post study survey.
Factors that can influence the duration of the illness include the age of the patients, presence of a second infection and use of other medications like antibiotics [2728]. However the primary aim of this study was to evaluate the tolerability of using a herbal preparation like YQS in our population of children with cross-reactive NSAID hypersensitivity. The study population has a propensity to develop drug hypersensitivity reactions, thus they may also develop hypersensitivity reactions to the herbal preparation.
Considering the availability of relatively safe and proven antipyretic medications for the general population of children, a study evaluating the feasilibity of a new medication for fever may not be medically and ethically warranted. However, in our population of children with cross-reactive NSAID hypersensitivity, where the use of safe and approved medications is not possible, an open label trial of a TCM in a controlled environment is a clinical and ethical imperative. We believe this is the first study evaluating YQS as a treatment for fever in upper respiratory tract infections. In our study, YQS has been found to be well tolerated with no severe adverse reactions. However the small number of patients studied prevents us from drawing further conclusions.
Limitations of our study include the small study cohort, and the postive biases towards acceptance and tolerance of TCM. While Eu Yan Sang provided the YQS, they had no influence on the design of the study and were not involved in patient evaluation or data analysis. In addition, the study was supported by a noncommercial grant provided by Singhealth Foundation.
In conclusion, YQS is generally well tolerated in patients with paracetamol and ibuprofen hypersensitivity and is potentially effective in reducing fever during an upper respiratory tract infection. Further clinical trials however are needed to confirm the potential therapeutic benefits of this herbal preparation. Even though reactions with this preparation are rare, in this subgroup of high risk patients, an observed challenge with the preparation prior to its home use seems to be warranted.
Figures and Tables
ACKNOWLEDGEMENTS
We would like to acknowledge the assistance of the respiratory technologists Tan Soh Gin, Tan Choy Hoon, Lim Mei Lan, Tan Yi Ping, Choo Pui Shan, He Qixian, and Teo Jia Hui Candice. We would also like to thank Eu Yan Sang for acting as an external advisor and providing the sachets of Yin Qiao San used in this study. All phases of this study were supported by a noncommercial grant provided by Singhealth Foundation (grant No. SHF/TCM001/2008). Singhealth Foundation and Eu Yan Sang had no influence on the design of the study and were not involved in patient evaluation or data analysis. The authors have no financial relationship relevant to this article to disclose. Chay Oh Moh is a member of the Eu Yan Sang scientific advisory board. The other authors have no potential conflicts of interest to disclose.
References
1. Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002; 99:13926–13931.

2. Roberts LJ, Morrow JD. Analgesic-antipyretic and antiinflammatory agents and drugs employed in the treatment of gout. In : Goodman LS, Hardman JG, Limbird LE, Gilman AG, editors. The pharmacological basis of therapeutics. 10th ed. New York: McGraw-Hill;2001. p. 687–731.
3. Szczeklik A, Nizankowska E, Sanak M. Hypersensitivity to aspirin and non-steroidal antiinflammtory drugs. In : Adkinson NF, Busse WW, Bochner BS, Holgate ST, Simons FE, Lemanske RF, editors. Middelton's allergy, principles and practice. 7th ed. Philadelphia: Mosby;2009. p. 1227–1243.
4. Tan VA, Gerez IF, Van Bever HP. Prevalence of drug allergy in Singaporean children. Singapore Med J. 2009; 50:1158–1161.
5. Jenkins C, Costello J, Hodge L. Systematic review of prevalence of aspirin induced asthma and its implications for clinical practice. BMJ. 2004; 328:434.

6. Boussetta K, Ponvert C, Karila C, Bourgeois ML, Blic J, Scheinmann P. Hypersensitivity reactions to paracetamol in children: a study of 25 cases. Allergy. 2005; 60:1174–1177.

7. Stevenson DD, Szczeklik A. Clinical and pathologic perspectives on aspirin sensitivity and asthma. J Allergy Clin Immunol. 2006; 118:773–786.

8. Kim YP, Lee EB, Kim SY, Li D, Ban HS, Lim SS, Shin KH, Ohuchi K. Inhibition of prostaglandin E2 production by platycodin D isolated from the root of Platycodon grandiflorum. Planta Med. 2001; 67:362–364.

9. Wang C, Schuller Levis GB, Lee EB, Levis WR, Lee DW, Kim BS, Park SY, Park E. Platycodin D and D3 isolated from the root of Platycodon grandiflorum modulate the production of nitric oxide and secretion of TNF-alpha in activated RAW 264.7 cells. Int Immunopharmacol. 2004; 4:1039–1049.
10. Wu HZ, Luo J, Yin YX, Wei Q. Effects of chlorogenic acid, an active compound activating calcineurin, purified from Flos Lonicerae on macrophage. Acta Pharmacol Sin. 2004; 25:1685–1689.
11. Cho MK, Jang YP, Kim YC, Kim SG. Arctigenin, a phenylpropanoid dibenzylbutyrolactone lignan, inhibits MAP kinases and AP-1 activation via potent MKK inhibition: the role in TNF-alpha inhibition. Int Immunopharmacol. 2004; 4:1419–1429.
12. Lin CC, Lu JM, Yang JJ, Chuang SC, Ujiie T. Anti-inflammatory and radical scavenge effects of Arctium lappa. Am J Chin Med. 1996; 24:127–137.

13. Yu LZ, Wu JY, Luo JB, Huang XG, Shao HX, Lin H. Experimental study on anti-pyretic effect of gegen qin lian decoction and its compounds. Zhongguo Zhong Yao Za Zhi. 2004; 29:663–666.
14. Jin UH, Lee JY, Kang SK, Kim JK, Park WH, Kim JG, Moon SK, Kim CH. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: isolation and identification from methanol extract of Euonymus alatus. Life Sci. 2005; 77:2760–2769.

15. Yuan X, Koh HL, Chui WK. A high performance liquid chromatography method for the simultaneous determination of arctiin, chlorogenic acid and glycyrrhizin in a Chinese proprietary medicine. J Pharm Biomed Anal. 2005; 39:697–704.

16. Yu BS, Yan XP, Xiong J, Xin Q. Simultaneous determination of chlorogenic acid, forsythin and arctiin in Chinese traditional medicines preparation by reversed phase-HPLC. Chem Pharm Bull (Tokyo). 2003; 51:421–424.

17. Kong XT, Fang HT, Jiang GQ, Zhai SZ, O'Connell DL, Brewster DR. Treatment of acute bronchiolitis with Chinese herbs. Arch Dis Child. 1993; 68:468–471.

18. Wu T, Chen X, Duan X, Juan N, Liu G, Qiao J, Wang Q, Wei J, Zhen J, Zhou L. Chinese medicinal herbs for acute bronchitis. Cochrane Database Syst Rev. 2005; (3):CD004560.

19. Chen XY, Wu TX, Liu GJ, Wang Q, Zheng J, Wei J, Ni J, Zhou LK, Duan X, Qiao JQ. Chinese medicinal herbs for influenza. Cochrane Database Syst Rev. 2005; (1):CD004559.

20. Kidon MI, Kang LW, Chin CW, Hoon LS, See Y, Goh A, Lin JT, Chay OM. Early presentation with angioedema and urticaria in cross-reactive hypersensitivity to nonsteroidal antiinflammatory drugs among young, Asian, atopic children. Pediatrics. 2005; 116:e675–e680.

21. Chay OM, Tang JPL, Gu K, Goh A, Phua KB, Lim WH. Use of herbal therapy among parents: a questionnaire survey. Singapore Paediatr J. 2001; 43:37–42.
22. Kidon MI, Liew WK, Chiang WC, Lim SH, Goh A, Tang JP, Chay OM. Hypersensitivity to paracetamol in Asian children with early onset of nonsteroidal anti-inflammatory drug allergy. Int Arch Allergy Immunol. 2007; 144:51–56.

23. Woessner KM, Simon RA, Stevenson DD. The safety of celecoxib in patients with aspirin-sensitive asthma. Arthritis Rheum. 2002; 46:2201–2206.

24. Martin-Garcia C, Hinojosa M, Berges P, Camacho E, Garci a-Rodriguez R, Alfaya T, Iscar A. Safety of a cyclooxygenase-2 inhibitor in patients with aspirin-sensitive asthma. Chest. 2002; 121:1812–1817.

25. Sanchez-Borges M, Capriles-Hulett A. Atopy is a risk factor for nonsteroidal anti-inflammatory drug sensitivity. Ann Allergy Asthma Immunol. 2000; 84:101–106.

26. Rachelefsky GS, Coulson A, Siegel SC, Stiehm ER. Aspirin intolerance in chronic childhood asthma: Detected by oral challenge. Pediatrics. 1975; 56:443–448.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download