Abstract
H1-antihistamine is generally a well-tolerated and safe drug. However, in resemblance with all other drugs, H1-antihistamines can also prompt adverse drug reactions (ADRs). We recently encountered the very unusual ADR of H1-antihistamine-induced gynecomastia. A 21-year-old man with idiopathic anaphylaxis was treated with ebastine (Ebastel), a second-generation H1-antihistamine, for the prevention of anaphylaxis. Three months later, the patient remained well without anaphylaxis, but had newly developed gynecomastia. Because anaphylaxis recurred after the cessation of H1-antihistamine, the preventive medication was changed to omalizumab. A few months later, his gynecomastia had entirely disappeared. Physicians should be aware of this exceptional ADR of H1-antihistamine.
H1-antihistamine is one of the most commonly used drugs in the world [1]. It offsets various histamine-mediated reactions as an inverse agonist of H1-receptor. H1-antihistamine is generally a well-tolerated and safe drug. Nonetheless, in resemblance with all other drugs, H1-antihistamines also prompt adverse drug reactions (ADRs). ADRs are mediated through diverse mechanisms: blocking of H1-receptor in the central nervous system (confusion), blocking of muscarinic receptor (dry mouth and urinary retention), blocking of α-adrenergic receptor (hypotension and reflex tachycardia), blocking of serotonin receptor (increased appetite and weight gain), and blocking of cardiac ion-channel (QT prolongation/arrhythmia). These ADRs are usually produced by first-generation H1-antihistamine, and are almost absent in association with the second-generation H1-antihistamines that we have commonly used in recent years [2].
Excepting the abovementioned ADRs, side effects of H1-antihistamine are very scarce. Rare side-effects include hypersensitivity reactions (including bronchospasm, angioedema, and anaphylaxis), convulsions, paresthesia, blood disorders, extrapyramidal effects, tremor, and liver dysfunction [3]. However, we recently encountered a case in which gynecomastia was induced by ebastine (Ebastel, Boryung Pharmaceutical Co., Seoul, Korea), a second-generation H1-antihistamine, in a patient with idiopathic anaphylaxis.
A 21-year-old man visited our outpatient clinic because of recurrent episodes of resting dyspnea, which had been present for 5 months. The dyspnea was not associated with exertion, but had instead been occurring during the nighttime with wheezing, cough, urticaria, and lip angioedema. Initially, the dyspnea and associated symptoms had occurred 1-2 times per week. More recently, however, they had been occurring every night and the patient could hardly sleep. He was a university student and a current, 1-pack-year smoker. The patient had mild perennial allergic rhinitis, but no history of drug allergy, asthma, or other chronic diseases. He denied exposure to any potentially inducing agents before the symptoms had developed. A review of systems and a physical examination at our daytime outpatient clinic did not reveal any symptom or sign of the condition that was bothering him nightly. His chest x-ray was normal. The results of a baseline pulmonary function test and bronchial provocation test (methacholine) were within normal limits. On allergic skin testing, only Dermatophagoides pteronyssinus was positive (3+). The results of other laboratory tests (complete blood count, chemistry, total immunoglobulin E, and tryptase) were all within normal limits.
He was diagnosed as having idiopathic anaphylaxis. Oral corticosteroid (prednisolone, 15 mg twice a day) and H1-antihistamine (ebastine, 10 mg twice a day) were prescribed as maintenance medications for the prevention of anaphylaxis. For emergency preparedness, an epinephrine auto-injector was also prescribed. Beginning on the first day of the maintenance medication, all the symptoms that had bothered the patient disappeared. After 1 month, prednisolone was tapered off, and only ebastine was used for the prevention of anaphylaxis. After 2 months of maintenance therapy, no anaphylaxis had occurred, and the dose of ebastine was therefore reduced to 10 mg once a day.
During 3 months of preventive therapy, the patient had been very well and had not experienced an anaphylactic attack. However, both of his breasts had newly become enlarged (Fig. 1A, B). Ultrasound showed abnormally proliferated subareolar glandular tissue in the breasts, which was compatible with gynecomastia (Fig. 1C, D). An endocrinologist recommended a hormonal study. The results were all within normal limits, although the prolactin level was high normal: prolactin, 18.74 ng/mL (normal reference, 1.61-18.77 ng/mL); testosterone, 5.78 ng/mL (normal reference, 2.41-8.27 ng/mL); estradiol, 37.44 pg/mL (normal reference, 5-4,300 pg/mL); thyroid-stimulating hormone, 1.45 µIU/mL (normal reference, 0.55-4.78 µIU/mL); luteinizing hormone, 2 (normal reference, 2-12). We concluded that the current medication (ebastine) was the probable culprit of the patient's gynecomastia.
At first, we changed the class of H1-antihistamine that was provided to the patient: ebastine (a class of piperidine) was stopped and cetirizine (a class of piperazine) was started. One month later, the gynecomastia had progressed further despite the new H1-antihistamine (cetirizine). We decided to stop the administration of H1-antihistamine because anaphylaxis had not occurred at any point during the preventive therapy with H1-antihistamine. A few weeks later, the patient's dyspnea, cough, wheezing, and urticarial/lip angioedema had relapsed and were worsening. His breasts had somewhat regressed. In the end, we started omalizumab (150 mg every 4 weeks) as a new preventive treatment for anaphylaxis. During the first few days after beginning omalizumab, the symptoms of anaphylaxis gradually subsided. His breasts also showed gradual regression. By approximately 6 months later, gynecomastia had completely disappeared. Further, the prolactin level had decreased to 8.91 ng/mL.
Gynecomastia is the benign proliferation of the glandular tissue of the male breast [4]. The major causes of gynecomastia are idiopathic (25%), puberty (25%), drugs (20%), and testicular tumors (3%) [5]. The main pathogenesis of gynecomastia is thought to be a relative increase in estrogen, as compared with androgen. Estrogen-excess and androgen-deficiency induce the proliferation of glandular tissue in the breast. Generally, clinically meaningful gynecomastia has developed when the breast glandular tissue exceeds 0.5 cm in men [5].
Once the diagnosis of gynecomastia has been established, a drug-review is critical [4]. Drugs are the most common cause of gynecomastia in adults, with the exception of idiopathic causes. The possibility of induction by drugs is important to consider because gynecomastia can resolve after cessation of the culprit drug. The pathogenesis that has been described above (estrogen-excess and androgen-deficiency) is also the main mechanism of most drug-induced gynecomastia, such as cases induced by neuroleptics, ketoconazole, metronidazole, spironolactone, cimetidine, metoclopramide, and other medications [6]. Nonetheless, isoniazid, proton pump inhibitors, and some other drugs have uncertain mechanisms [6].
H1-antihistamines have rarely been reported as culprits of gynecomastia. During a literature review, we found only one case report of H1-antihistamine-induced gynecomastia [7]. In the report, the culprit of gynecomastia was identified to be cetirizine, which is a class of piperazine and a second-generation H1-antihistamine. In our case, ebastine (a class of piperidine) was the initial culprit and cetirizine was involved in further progression. Ebastine has never been reported as a cause of gynecomastia. In our case, we attempted to resolve gynecomastia by switching the class of H1-antihistamine; however, this attempt failed.
The mechanism by which H1-antihistamine causes gynecomastia is explained by the structural similarity of neuroleptics and H1-antihistamines [7]. Neuroleptic drugs block dopamine receptor in the pituitary gland, thus interrupting normal dopaminergic inhibition of prolactin secretion. In this manner, neuroleptics could increase the level of prolactin. In men, hyperprolactinemia induces hypogonadotropic hypogonadism (androgen-deficiency), and the resulting relative increases in estrogen could lead to gynecomastia [8]. In the earlier case report, hyperprolactinemia was noted in patients with gynecomastia. In our case, the patient also had a relatively increased level of prolactin [7]. Eventually, prolactin levels have reduced in all patients after the regression of gynecomastia.
In conclusion, we have reported a case in which ebastine (Ebastel), a second-generation H1-antihistamine, induced gynecomastia in a patient with idiopathic anaphylaxis. Accordingly, we suggest that H1-antihistamine could be a culprit of gynecomastia. Physicians should be aware of this exceptional ADR of H1-antihistamine.
Figures and Tables
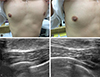 | Fig. 1(A) Grossly enlarged breasts were noted on the anterior chest. (B) The right breast enlargement was more prominent than the left breast enlargement. Ultrasound showed abnormally proliferated subareolar glandular tissue in the right (C) and left (D) breasts, which was compatible with gynecomastia. |
References
2. Simons FE, Simons KJ. Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol. 2011; 128:1139–1150.e4.

3. Scadding GK. Clinical assessment of antihistamines in rhinitis. Clin Exp Allergy. 1999; 29:Suppl 3. 77–81.

4. Braunstein GD. Clinical practice: gynecomastia. N Engl J Med. 2007; 357:1229–1237.
6. Cuhaci N, Polat SB, Evranos B, Ersoy R, Cakir B. Gynecomastia: clinical evaluation and management. Indian J Endocrinol Metab. 2014; 18:150–158.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download