Abstract
Tacrolimus and cyclosporine have been used in various formulations, but their hypersensitivity reactions are rare in practice. Castor oil derivatives are nonionic surfactants used in aqueous preparations of hydrophobic active pharmaceutical ingredients. Castor oil derivatives that can be used as additives to tacrolimus and cyclosporine may play a role in the development of hypersensitivity reactions, especially anaphylaxis. Various immunologic and nonimmunologic mechanisms have been implicated in hypersensitivity reactions induced by castor oil derivatives. Physicians should be aware that not only the drug itself, but also its additives or metabolites could induce hypersensitivity reactions. We report a case of anaphylaxis caused by vitamin K (phytonadine), serotonin antagonist (granisetron), intravenous tacrolimus, and cyclosporine. Interestingly, the patient tolerated oral cyclosporine, which did not contain Cremophor EL or polysorbate 80.
Tacrolimus and cyclosporine have been approved for the prevention of graft versus-host disease (GVHD), which is the major cause of morbidity and mortality after allogeneic bone marrow transplantation. Tacrolimus, FK 506, is an immunosuppressive drug that prevents GVHD by inhibiting calcineurin [1]. Tacrolimus is prescribed as a substitute for cyclosporine in unrelated-donor transplantation. Cyclosporine is a potent immunosuppressant widely used to block the transcription of cytokine genes in activated T cells [2]. Anaphylactic reactions are rare side effects of both tacrolimus and cyclosporine therapy. We report a case of anaphylaxis in a patient with acute myeloid leukemia after infusion of intravenous tacrolimus and cyclosporine. Cremophor, also known as polyethoxylated castor oil, was suspected to induce the reaction.
A 47-year-old male patient with acute myeloid leukemia, after undergoing induction and consolidation chemotherapy, was admitted for allogeneic bone marrow transplantation from an unrelated donor. He had a medical history of peach-induced urticaria, multivitamin concentration-induced urticaria, and anaphylactic reaction probable to vitamin K1 (phytonadine) and serotonin antagonist (granisetron). Conditioning therapy with fludarabine, busulfan, and antithymocyte globulin were initiated for 6 days and intravenous tacrolimus (Prograf, Astellas Pharma, Tokyo, Japan) was started 1 day before allogeneic bone marrow transplantation at a dose of 0.03 mg/kg over 24-hour continuous infusion. He began to complain of nausea, abdominal bloating, chest tightness, dizziness, conjunctival injection, and whole body itching progressing to generalized urticaria 1 hour after the administration of tacrolimus. His vital signs were as follows: blood pressure, 86/50 mm Hg; pulse rate, 103/min; respiratory rate, 17/min; and body temperature, 36.8℃. Administration of tacrolimus was immediately stopped. He received systemic corticosteroid followed by intravenous hydration, antihistamine, and H2 antagonist. His complaints resolved after treatment. Tacrolimus was then switched to intravenous cyclosporine (Sandimmun, Novartis, Basel, Switzerland) at a dose of 1.5 mg/kg over 24-hour continuous infusion. Within 10 minutes after beginning cyclosporine administration, he complained of nausea, abdominal bloating, and chest tightness. The vital signs were as follows: blood pressure, 78/47 mm Hg; pulse rate, 151/min; respiratory rate, 26/min; and body temperature, 36.4℃. Infusion of cyclosporine was discontinued and symptoms disappeared spontaneously. The patient was consulted to an allergist. Intravenous cyclosporine and tacrolimus were considered as the causative drugs of anaphylaxis. Particularly, the solubilizing vehicle, polyethoxylated castor oil, was suspected to be the causative agent rather than tacrolimus or cyclosporine itself. Because alternative treatment was needed for GVHD prevention in this patient, oral capsules of cyclosporine (Sandimmun Neoral, Novartis) that did not contain the causative polyethoxylated castor oils, Cremophor EL and Cremophor RH 60, were administered by 3-dose graded challenge (25 mg, 50 mg, and 100 mg with 2-hour intervals). Oral cyclosporine (Sandimmun Neoral) did not cause a significant reaction and was administered at 150 mg twice daily. The patient received bone marrow mononuclear cells that had been harvested from an unrelated donor with successful engraftment. We achieved dosage adjustment by monitoring cyclosporine blood levels. The patient was discharged without acute GVHD on day 15 and was taking 150- to 120-mg cyclosporine daily.
Polyethoxylated castor oil is a nonionic solubilizer and emulsifier prepared by reacting varying amounts of ethylene oxide with either castor oil or hydrogenated castor oil. The Cremophor series (Cremophor EL, Cremophor RH 40, Cremophor RH 60, etc.) are well-known among the several available materials [3]. Polyethoxylated castor oil is particularly useful as a vehicle for a variety of hydrophobic drugs. Because of its properties, polyethoxylated castor oil has been widely used to improve the solubility of water-insoluble drugs such as anticancer, immunosuppressive, analgesic, anesthetic drugs, vitamins and new synthetic water-insoluble compounds. The various formulations available containing polyethoxylated castor oil are summarized in Table 1. Many studies have suggested that polyethoxylated castor oil plays role in the hypersensitivity reaction to formulations since the first report of a polyethoxylated castor oil hypersensitivity reaction in 1974 [4]. Several hypersensitivity reactions to tacrolimus as well as cyclosporine presented with pruritus, rash, urticaria, and angioedema progressing to anaphylaxis at various doses and formulations. Among patients reported to have allergic reactions to a parenteral form of both tacrolimus or cyclosporine, very few allergic reactions were attributed to the Castor oil derivatives: Cremophor RH 60 (polyoxyl 60 hydrogenated castor oil) and Cremophor EL (polyoxyl 35 hydrogenated castor oil) [567]. Cremophor RH 60 is a component of intravenous tacrolimus and is a polyethoxylated castor oil similar to Cremophor EL, which is a component of intravenous cyclosporine. Although our patient had never exposed to intravenous tacrolimus and cyclosporine, he developed anaphylactic reactions to both drugs. Serial episodes revealed that administered polyethoxylated castor oil was most likely responsible for the anaphylaxis, and he may have exposed to other castor oil derivatives previously. Our patient also experienced urticaria due to multivitamin concentration and then anaphylactic reaction due to vitamin K1 (phytonadione). Polyethoxylated castor oil was first considered the cause of anaphylaxis, because the patient had a medical history of anaphylactic reaction due to vitamin K1 (phytonadione) that might contain polyethoxylated castor oil. However, polysorbate 80 is used as a base for phytonadione rather than polyethoxylated castor oil in Korea. Not only phytonadione, a fat-soluble synthetic vitamin K1 analog, but also multivitamin concentration as well as a serotonin antagonist such as granisetron (Kytril, Roche, Basel, Switzerland) contain polysorbate 80 as a nonmedicinal ingredient. Polysorbate 80 is a nonionic surfactant derived from polyethylene glycol-ylated sorbitan esterified with fatty acids. Polysorbate 80 commonly used in medicinal formulations and food products may cause hypersensitivity reactions in some patients. The various formulations available containing polysorbate 80 are summarized in Table 2. This patient had a hypersensitivity reaction to polysorbate 80 as well. Cremophor and polysorbate have similar functional and structural components. They comprise a complex mixture of hydrophobic and hydrophilic molecules produced by a polyethoxylation reaction. There have been previous cases of a hypersensitivity reaction between cremophor and polysorbate-containing drug [8] and other reported possible cross-reactivity for each surfactant, which could result in complement activation [9]. Several mechanisms related to polyethoxylated castor oil-induced hypersensitivity have been studied, including an IgE-mediated reaction [10], complement activation [11] and histamine release [12] by mast cell or basophil. A positive skin test or basophil activation test with previous exposure of drugs suggests IgE-mediated reactions. Evidence supporting a role for complement activation following the first exposure to drugs derives from histamine release.
For those with serious hypersensitivity reactions caused by intravenous tacrolimus and cyclosporine, it is important to know what could be alternatives. Formulations that do not contain castor oil derivatives appear to be a safe alternative. Oral formulations are available as a capsule or oral solution. However, anaphylactic reactions have been reported for the traditional oral solution (Sandimmun, Novartis), which contains olive oil with polyethoxylated oleic glycerides [1314]. The traditional soft gelatin capsule (Sandimmun) contains corn oil with polyethoxylated glycolysed glycerides and may be the drug of choice for anaphylaxis with intravenous cyclosporine. Both the microemulsion form of the oral solution and the soft gelatin capsule contain corn oil with Cremophor RH 40 (Polyoxyl 40 hydrogenated castor oil) which may be allergenic in a patient with a reaction to the intravenous formulation [15]. However, in our case, the patient tolerated oral cyclosporine containing Cremophor RH 40 though an anaphylactic reaction to polyethoxylated castor oils, Cremophor RH 60 and Cremophor EL, as a surfactant ingredient of intravenous tacrolimus and cyclosporine, respectively. A previous study reported that a hypersensitivity reaction to one formulation of cyclosporine did not preclude the use of another formulation [715]. Further studies are needed to evaluate hypersensitivity reactions caused by polyethoxylated castor oils in order to investigate the possible cross-reactivity among these surfactants.
In conclusion, we present a rare case of anaphylaxis caused by both intravenous tacrolimus and intravenous cyclosporine but not by oral cyclosporine. Polyethoxylated castor oil was suspected to be the causative agent. Our case indicates that we should make a careful consideration not only to the drug itself, but also to its additives or metabolites particularly in patients with histories of multiple drug hypersensitivity reactions. Anaphylactic events by drugs containing castor oil derivatives are usually unavoidable despite decreasing the infusion rate, proper mixing, and premedication [16]. When an anaphylactic reaction is recognized, optimal treatment should begin with stopping the culprit medication immediately and intramuscular injection of epinephrine. Choosing a different formulation of drugs that do not contain the causative castor oil derivatives is a safe strategy.
Figures and Tables
Table 2
Drug list containing polysorbate 80
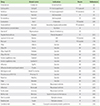
IV, intravenous; PO, peroral; IM, intramuscular; SC, subcutaneous; HIV, human immunodeficiency virus; DTap, diphtheria, tetanus, and acellular pertussis; IPV, inactivated polio vaccine; HePB, hepatitis B ; Hib, Haemophilus influenzae type B; PCV, pneumococcal conjugate vaccine.
*Torisel kit: Torisel injection supplied with diluent for torisel which contains polysorbate 80.
†Formulation with Cremophor EL is available on the market.
References
1. Fay JW, Wingard JR, Antin JH, Collins RH, Pineiro LA, Blazar BR, Saral R, Bierer BE, Przepiorka D, Fitzsimmons WE, Maher RM, Weisdorf DJ. FK506 (Tacrolimus) monotherapy for prevention of graft-versus-host disease after histocompatible sibling allogenic bone marrow transplantation. Blood. 1996; 87:3514–3519.

2. Ringden O. Cyclosporine in allogeneic bone marrow transplantation. Transplantation. 1986; 42:445–452.

3. Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res. 2004; 21:201–230.

4. Kessell J, Assem ES. Letter: An adverse reaction to althesin. Br J Anaesth. 1974; 46:209.
5. Nicolai S, Bunyavanich S. Hypersensitivity reaction to intravenous but not oral tacrolimus. Transplantation. 2012; 94:e61–e63.

6. Takamatsu Y, Ishizu M, Ichinose I, Ogata K, Onoue M, Kumagawa M, Suzumiya J, Tamura K. Intravenous cyclosporine and tacrolimus caused anaphylaxis but oral cyclosporine capsules were tolerated in an allogeneic bone marrow transplant recipient. Bone Marrow Transplant. 2001; 28:421–423.

7. Volcheck GW, Van Dellen RG. Anaphylaxis to intravenous cyclosporine and tolerance to oral cyclosporine: case report and review. Ann Allergy Asthma Immunol. 1998; 80:159–163.

8. Friedland D, Gorman G, Treat J. Hypersensitivity reactions from taxol and etoposide. J Natl Cancer Inst. 1993; 85:2036.

9. Weiszhár Z, Czucz J, Revesz C, Rosivall L, Szebeni J, Rozsnyay Z. Complement activation by polyethoxylated pharmaceutical surfactants: Cremophor-EL, Tween-80 and Tween-20. Eur J Pharm Sci. 2012; 45:492–498.

10. Ebo DG, Piel GC, Conraads V, Stevens WJ. IgE-mediated anaphylaxis after first intravenous infusion of cyclosporine. Ann Allergy Asthma Immunol. 2001; 87:243–245.

11. Szebeni J. Complement activation-related pseudoallergy: a new class of drug-induced acute immune toxicity. Toxicology. 2005; 216:106–121.

12. Gaudy JH, Sicard JF, Lhoste F, Boitier JF. The effects of cremophor EL in the anaesthetized dog. Can J Anaesth. 1987; 34:122–129.

13. Cooney GF, Alpern JB, Narins BE, Goetz LK, Cavarocchi NC. Tolerance of cyclosporine oral capsules in a patient hypersensitive to standard oral and intravenous solutions of the drug. Transplantation. 1990; 49:823–824.

14. van Hooff JP, Bessems P, Beuman GH, Leunissen KM. The absence of an allergic reaction to cyclosporine capsules in a patient allergic to standard oral and intravenous solutions of cyclosporine. Transplant Proc. 1988; 20:2 Suppl 2. 640.
15. Kuiper RA, Malingré MM, Beijnen JH, Schellens JH. Cyclosporine-induced anaphylaxis. Ann Pharmacother. 2000; 34:858–861.

16. Michaud LB. Methods for preventing reactions secondary to Cremophor EL. Ann Pharmacother. 1997; 31:1402–1404.

17. Bristol-Myers Squibb. Prescribing information for Ixempra kit (ixabepilone) Injection 2011 [Internet]. Princeton (NJ): Bristol-Myers Squibb;c2015. cited 2015 Jul 10. Available from: http://packageinserts.bms.com/pi/pi_ixempra.pdf.
18. AbbVie. Prescribing information for Kaletra (Lopinavir and Ritonavir) tablets and oral solution [Internet]. North Chicago (IL): AbbVie;c2015. cited 2015 Jul 10. Available from: http://www.rxabbvie.com/pdf/kaletratabpi.pdf.
19. AbbVie. Prescribing information for Norvir (ritonavir) soft gelatin capsules, tablets and oral solution [Internet]. North Chicago (IL): AbbVie;c2015. cited 2015 Jul 10. Available from: http://norvir.com/important-safety-information.cfm.
20. Hospira. Phytonadione (vitamin K1) injectable emulsion safety data sheet [Internet]. Lake Forest (IL): Hospira;c2015. cited 2015 Jul 10. Available from: http://www.hospira.com/en/search?q=phytonadione&fq=on.
21. Novartis AG. Package leaflet: information for the user for Tegretol [Internet]. Basel (CH): Novartis AG;c2015. cited 2015 Jul 10. Available from: http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1412572274774.pdf.
22. Novartis Pharmaceuticals UK Ltd. Package leaflet: information for the user for Anafranil [Internet]. West Sussex (UK): Novartis Pharmaceuticals UK Ltd.;c2015. cited 2015 Jul 10. Available from: http://www.medicines.org.uk/emc/PIL.21096.latest.pdf.
23. Wyeth Pharmaceuticals Inc.a subsidiary of Pfizer Inc.Prescribing information for Torisel (temsirolimus) kit Injection [Internet]. New York: Pfizer;c2015. cited 2015 Jul 10. Available from: http://labeling.pfizer.com/showlabeling.aspx?id=490.
24. Hoffmann-La Roche Ltd.Consumer information for Kytril (granisetron) tablets [Internet]. Mississauga (ON): Hoffmann-La Roche Ltd.;c2015. cited 2015 Jul 10. Available from: http://www.rochecanada.com/content/dam/internet/corporate/rochecanada/en_CA/documents/Research/ClinicalTrialsForms/Products/ConsumerInformation/MonographsandPublicAdvisories/Kytril/Kytril_PM_CIE.pdf.
25. Genentech Inc. Prescribing information for Pegasys (peginterferon alfa-2a) Injection [Internet]. San Francisco (CA): Genentech Inc.;c2015. cited 2015 Jul 10. Available from: http://www.gene.com/download/pdf/pegasys_prescribing.pdf.
26. Wolters Kluwer Health. Vaccine excipient and media summary (CDC). St Louis (MO): Wolters Kluwer Health;c2015. cited 2015 Jul 10. Available from: http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/B/excipient-table-2.pdf.
27. AbbVie. Prescribing information for Humira (adalimumab) [Internet]. North Chicago (IL): AbbVie;c2015. cited 2015 Jul 10. Available from: http://www.rxabbvie.com/pdf/humira.pdf.
28. Janssen Biotech Inc. Prescribing information for Remicade (infliximab) [Internet]. Horsham (PA): Janssen Biotech Inc.;c2015. cited 2015 Jul 10. Available from: http://www.remicade.com/shared/product/remicade/prescribing-information.pdf.
29. Genentech Inc. Prescribing information for Actemra (tocilizumab) [Internet]. San Francisco (CA): Genentech Inc.;c2015. cited 2015 Jul 10. Available from: http://www.gene.com/download/pdf/actemra_prescribing.pdf.
30. Human Genome Sciences, Inc. Prescribing information for Benlysta (belimumab) [Internet]. Rockville (MD): Human Genome Sciences Inc., a subsidiary of GlaxoSmithKline;c2015. cited 2015 Jul 10. Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Benlysta/pdf/BENLYSTA-PI-MG.PDF.
31. Genzyme Corp. Prescribing information for Lemtrada (alemtuzumab) [Internet]. Cambridge (MA): Genzyme Corp.;c2015. cited 2015 Jul 10. Available from: http://products.sanofi.us/lemtrada/lemtrada.pdf.
32. Biogen Idec Inc. Prescribing information for Tysabri (natalizumab) [Internet]. Cambridge (MA): Biogen Idec Inc.;c2015. cited 2015 Jul 10. Available from: http://www.tysabri.com/prescribingInfo.
33. Novartis Pharmaceuticals Corp. Prescribing information for Ilaris (canakinumab) [Internet]. East Hanover (NJ): Novartis Pharmaceuticals Corp.;c2015. cited 2015 Jul 10. Available from: http://www.pharma.us.novartis.com/product/pi/pdf/ilaris.pdf.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download