Abstract
Chlorpheniramine is a widely prescribed H1-antihistamine for relieving urticaria or histamine-mediated allergic reactions. However, although rare, it may cause immediate hypersensitivity reactions. The diagnosis is usually made by provocation test, but its application is often limited due to comorbidities or potential risk of severe reactions. In those cases, skin tests and basophil activation tests can be considered as additional diagnostic tests for the drug allergy. Here, we report a 33-year-old female with underlying chronic urticaria, who recurrently developed anaphylaxis after chlorpheniramine administration. Intradermal test showed positive responses in the patient at 0.02 mg/mL of chlorpheniramine, but not in healthy controls. Basophil activation test showed significant up-regulation of CD63 and CD203c by chlorpheniramine. The present case reminds the rare but potential allergic risk of chlorpheniramine, and also suggests the potential utility of basophil activation test in making the diagnosis.
Chlorpheniramine is one of the most classical H1-antihistamines, primarily prescribed for histamine-mediated hypersensitivity reactions such as urticaria. In the literature, cases have been rarely reported for chlorpheniramine-induced immediate hypersensitivity reactions [12], even when including pheniramine [3] or dexchlorpheniramine [456]. The mechanisms are still unclear, but IgE-mediated mechanisms have been suggested. The diagnosis is made by a drug provocation test (DPT), but may be supported by skin tests or basophil activation test (BAT) in cases needed. BAT is a safe and useful in vitro test [7], and the clinical application is expanding in diagnosing drug hypersensitivity [8]. Here we report a case of chlorpheniramine-induced anaphylaxis, of which diagnosis has been supported by skin tests and BAT.
A 33-year-old female with underlying chronic urticaria developed anaphylaxis after intravenous chlorpheniramine injection. She had visited the Emergency Department for acute flare-up of urticaria, and had been given intravenous chlorpheniramine. However, about 10 minutes later, she suddenly developed abdominal discomfort, urticarial aggravation, dizziness and hypotension (blood pressure, 72/44 mm Hg). The reactions resolved after treatments with epinephrine, corticosteroids and saline hydration. Any previous history was denied regarding prior food allergy, syncope or hypotensive episodes. At discharge, she was prescribed oral medications including prednisolone, levocetirizine and chlorpheniramine. However, she redeveloped dizziness and urticarial aggravation immediately after taking the medications, and thus attended the allergy clinic for further investigations.
In allergen skin prick tests (SPTs) and multiple allergosorbent test system analyses, only house dust mites and dog allergens showed positive responses. None of tested food allergens were positive. Serum total IgE level was 113 kU/L. Autologous serum skin test was negative. Electrocardiogram and chest x-ray were normal.
Oral provocation test was considered to determine the causal relationship with chlorpheniramine, but it could not be carried out due to the patient's personal reasons. Therefore, skin tests were performed for H1-antihistamines that she had recently taken. No skin reactions developed with levocetirizine or fexofenadine (prick and intradermal concentrations: 1 mg/mL for levocetirizine and 2.5 mg/mL for fexofenadine). No skin responses developed in SPT for 2 mg/mL chlorpheniramine maleate; however, positive intradermal test (IDT) responses were elicited at 1:100 dilution (increased wheal by 3 mm × 3.5 mm). In healthy volunteers (n = 4), only slight flare (2 mm × 2 mm) but no wheal reactions developed at the 1:100 dilution (0.02 mg/mL). The original concentration of chlorpheniramine increased wheal sizes by ≥3 mm in both of patient and healthy controls.
The relationship with chlorpheniramine was further assessed by BAT, using a commercially available Flow-CAST highsens kit according to the manufacturer's instructions (Bühlmann Laboratories AG, Schönenbuch, Switzerland). Briefly, the Flow-CAST highsens determines the basophil activation by measuring the relative amount of CD63 and CD203c positive basophils among the total population of CCR3 positive basophils in whole blood [9]. We performed the BAT using two forms of chlorpheniramine (0.1 mg/mL); a raw form and a conjugated form with albumin. The conjugation process was carried out overnight before the BAT day, using Imject PharmaLink Immunogen Kit, according to the manufacturer's instructions (Pierce, Rockford, IL, USA). Briefly, the kit facilitates the coupling process between the drug and bovine serum albumin, via the Mannich reaction using formaldehyde. In the molecular structure of chlorpheniramine, a single hydrogen associated with the aryl carbon atom is likely to react in the Mannich reaction. The expression of activation markers was 38.5% by negative control, and 92.5% by positive control (anti-FcεRI monoclonal antibody). Interestingly, we found that the expression of basophil activation markers (CD63 or CD203c) was increased by the conjugated-chlorpheniramine (59.9%), but not by chlorpheniramine (38.1%) or albumin (42.2%) alone (Fig. 1). On the basis of her recurrent history and the investigation results, we diagnosed the patient to have chlorpheniramine-induced anaphylaxis.
The present report describes a case with chlorpheniramine-induced anaphylaxis. The diagnosis was made on the basis of recurrent history, and supplemented by skin tests and BAT.
In general, DPT is necessary to identify a culprit drug. However, the test is potentially harmful, and thus the risk-benefit needs to be carefully assessed [10]. Recently, the diagnostic roles of skin tests or in vitro tests have been focused for drug allergy [7811]. They may not assess the causal relationships but only the sensitization, but have potentially wider clinical applications for their better safety. In the literature, several tests have been utilized for chlorpheniramine allergy [123456]. Oral or intramuscular provocation tests were performed in four cases [125], whereas skin tests were used in three cases [346].
Despite that the precise mechanisms of chlorpheniramine allergy are unknown, skin tests and BAT could have potential diagnostic utility for the immediate hypersensitivity reactions. The first discussion point is the utility of skin tests in chlorpheniramine hypersensitivity. SPT and IDT are used for evaluating immediate allergic reactions; however, it is necessary to determine maximum nonirritating test concentration for excluding false positivity, particularly in IDT [11]. We found that chlorpheniramine has a potential irritating property at the concentration of 2 mg/mL, which is the usual concentration for intravenous administration. Therefore, we determined the skin test positivity at the 100-fold diluted concentration (0.02 mg/mL), which did not increase wheals in four healthy controls but only in the patient. The findings indicate that a skin irritating potential of chlorpheniramine should be considered in skin testing. In the literature, dexchlorpheniramine was reported as nonirritating at 1/100 dilution [6], but chlorpheniramine has not been reported in controls.
Another point is the performance of BAT. Until now, the clinical application of BAT in drug hypersensitivity has been limited to a few drugs such as beta-lactams, neuromuscular blocking agents, nonsteroidal anti-inflammatory drugs, or radiocontrast media [8]. One case has been reported for dexchlorpheniramine [4], but not for chlorpheniramine yet. We suppose that BAT has a potential utility for particular drugs which have skin irritating potency like fluoroquinolones [12]. Interestingly, we found that chlorpheniramine increased in vitro basophil activation particularly when conjugated with albumin. The findings should be further evaluated, and the immunogenic or haptenic structure should be clarified. However, there are recent reports which assessed the basophil activation by protein bound drugs [1314]. In this regard, we suggest that the conjugation with albumin might be a possible option when considering BAT for chlorpheniramine.
Drug allergens usually induce lower activation% than inhalant or food allergens [15]. Therefore, the cutoff for drug BAT has been empirically recommended as the stimulation index (SI = allergen stimulation per negative control) ≥ 2. In this respect, it may be argued that the present case did not show positive results to chlorpheniramine (SI, 1.56). However, the patient showed enhanced baseline basophil activation (38.5% by negative control), which is more than 10 folds higher than the expected levels of 2-2.5%. We presume that the enhanced basophil activation was attributed to her underlying disorder-chronic urticaria, as the smaller findings were previously reported in those patients [16]. Collectively, we interpreted the basophil activation results (59.9% and SI 1.56 by the conjugated chlorpheniramine) to be positive.
Chlorpheniramine has been prescribed for more than several decades, and is still widely used for relieving urticaria or histamine-related reactions. Physicians should be aware that chlorpheniramine could occasionally be a cause for allergic reactions or fatal anaphylaxis. In addition, we suggest that BAT could be considered as a supplementary test for diagnosing chlorpheniramine allergy.
Figures and Tables
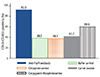 | Fig. 1Basophil activation tests with chlorpheniramine. The bar results indicate the percentage of basophil activation markers CD63/CD203c after stimulation with positive control (anti-FcεRI monoclonal antibody), negative control (buffer), chlorpheniramine, carrier protein, or chlorpheniramine conjugated with carrier protein. |
ACKNOWLEDGEMENTS
The study was supported by the grant A092076 from the Korea Healthcare Technology R & D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea.
References
1. Kim MH, Lee SM, Lee SH, Kwon HS, Kim SH, Cho SH, Min KU, Kim YY, Chang YS. A case of chlorpheniramine maleate-induced hypersensitivity with aspirin intolerance. Allergy Asthma Immunol Res. 2011; 3:62–64.

2. Lee SH, Jung HS, Yoon TY, Chang EJ, Kim MK, Kim KS. Allergic reaction to chlorpheniramine in a patient with aspirin-intolerant asthma. Korean J Asthma Allergy Clin Immunol. 2010; 30:55–58.
3. Kim YJ, Choi JH, Bang JS, Suh MK, Lee JW, Kim TH. A case of pheniramine maleate - aggravated chronic urticaria. Korean J Dermatol. 2000; 38:1414–1415.
4. Caceres Calle O, Fernandez-Benitez M. Allergy to dexchlorpheniramine: study of a case. Allergol Immunopathol (Madr). 2004; 32:306–309.
5. Rodríguez del Río P, Gonzalez-Gutierrez ML, Sanchez-Lopez J, Nunez-Acevedo B, Bartolome Alvarez JM, Martinez-Cocera C. Urticaria caused by antihistamines: report of 5 cases. J Investig Allergol Clin Immunol. 2009; 19:317–320.
6. Thurot-Guillou C, Bourrain JL, Jacquier JP, Beani JC. Anaphylactic reaction to ranitidine and dexchlorpheniramine. Eur J Dermatol. 2007; 17:170–171.
7. Romano A, Torres MJ, Castells M, Sanz ML, Blanca M. Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol. 2011; 127:3 Suppl. S67–S73.

8. Song WJ, Chang YS. Recent applications of basophil activation tests in the diagnosis of drug hypersensitivity. Asia Pac Allergy. 2013; 3:266–280.

9. McGowan EC, Saini S. Update on the performance and application of basophil activation tests. Curr Allergy Asthma Rep. 2013; 13:101–109.

10. Aberer W, Bircher A, Romano A, Blanca M, Campi P, Fernandez J, Brockow K, Pichler WJ, Demoly P. European Network for Drug Allergy (ENDA). EAACI interest group on drug hypersensitivity. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003; 58:854–863.

11. Brockow K, Garvey LH, Aberer W, Atanaskovic-Markovic M, Barbaud A, Bilo MB, Bircher A, Blanca M, Bonadonna B, Campi P, Castro E, Cernadas JR, Chiriac AM, Demoly P, Grosber M, Gooi J, Lombardo C, Mertes PM, Mosbech H, Nasser S, Pagani M, Ring J, Romano A, Scherer K, Schnyder B, Testi S, Torres M, Trautmann A, Terreehorst I. ENDA/EAACI Drug Allergy Interest Group. Skin test concentrations for systemically administered drugs: an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2013; 68:702–712.
12. Empedrad R, Darter AL, Earl HS, Gruchalla RS. Nonirritating intradermal skin test concentrations for commonly prescribed antibiotics. J Allergy Clin Immunol. 2003; 112:629–630.

13. Steiner M, Harrer A, Lang R, Schneider M, Ferreira T, Hawranek T, Himly M. Basophil activation test for investigation of IgE-mediated mechanisms in drug hypersensitivity. J Vis Exp. 2011; (55):pii: 3263.

14. Mayorga C, Andreu I, Aranda A, Dona I, Montanez MI, Blanca-Lopez N, Ariza A, Nuin E, Blanca M, Miranda MA, Torres MJ. Fluoroquinolone photodegradation influences specific basophil activation. Int Arch Allergy Immunol. 2013; 160:377–382.

15. De Week AL, Sanz ML, Gamboa PM, Aberer W, Bienvenu J, Blanca M, Demoly P, Ebo DG, Mayorga L, Monneret G, Sainte Laudy J. Diagnostic tests based on human basophils: more potentials and perspectives than pitfalls II Technical issues. J Investig Allergol Clin Immunol. 2008; 18:143–155.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download