Abstract
Background
It was previously reported that there is a positive correlation between incidence of type 1 diabetes and prevalence of asthma and atopic eczema. A negative correlation between the prevalence of type 1 diabetes and mortality from infectious diseases as well as a positive correlation with antibiotic susceptibility at a country level have also been reported.
Objective
The aim of this study was to investigate the association between country prevalence of rhinitis, atopic eczema, rhinoconjunctivitis, and wheezing with mortality from infectious diseases and also with antibiotic susceptibility at a country level.
Methods
Data for prevalence of rhinitis, eczema, rhinoconjunctivitis, and wheezing was obtained from the International Study of Asthma and Allergies in Childhood study (ISAAC). ISAAC Phase one was a multicentre multicountry cross sectional study involving over 700,000 children in 2 age groups of school children, 13-14 years old (adolescents) and 6-7 years old (children) in 156 centres from 56 countries. Mortality from infectious diseases was taken from World Health Organisation data. The Alexander project was used to identify antibiotic susceptibilities to common bacteria.
Results
There were significant positive correlations between atopic eczema and mortality from all infectious diseases studied, diarrhoeal illness, tropical infections, and childhood infections. A negative correlation exists between the prevalence of rhinitis and Streptococcus pneumoniae susceptibility to penicillin and to erythromycin, rhinitis and Haemophilus influenzae susceptibility to ampicillin and between rhinoconjunctivitis and H. influenzae susceptibility to ampicillin.
Atopic eczema is a chronic inflammatory skin condition that appears to be potentially associated with genetic and acquired defects in the proteins and lipids in the outer layer of the epidermis. This leads to a breakdown in the barrier between the internal body structures and the environment. Eczema is often linked to other atopic conditions including asthma and allergic rhinitis and rhinoconjunctivitis [1]. It affects approximately 5% to 20% of children worldwide [2] and the incidence appears to be on the increase. It appears to be higher in urban areas and developed countries, especially Western societies [34].
Our group has previously reported a correlation between incidence of type 1 diabetes and prevalence of asthma and atopic eczema [5]. The 'hygiene hypothesis' proposes that lack of microbial exposure in early postnatal life predisposes to certain immune-mediated diseases. It has been implicated in the pathogenesis of both type 1 diabetes [6] as well as in the pathogenesis of atopic diseases such as atopic eczema and asthma [7]. Lack of microbial exposure may therefore explain the observed correlation between the incidence of type 1 diabetes and prevalence of atopic disease. It has been suggested that infectious disease mortality and antibiotic susceptibility may be good markers of infective burden at a population level [8]. We therefore sought to investigate the possible relationship between the country prevalence of rhinitis, atopic eczema, rhinoconjunctivitis, and wheezing as documented in the International Study of Asthma and Allergies in Childhood study (ISAAC) Phase one study [9] to mortality from infectious diseases as reported by the World Health Organization (WHO) [1011] and to antibiotic susceptibility data for Streptococcus pneumoniae and Haemiophilus influenzae as reported in the Alexander project [12]. It should be noted that the prevalence of type 1 diabetes has already been reported to be negatively correlated with mortality from infectious diseases and positively with antibiotic susceptibility at a country level [8].
The ISAAC involved three phases. Phase one being carried out between 1994 and 1995 with 156 collaborating centres in 56 countries with a total of 721,601 children participating. This phase showed a variation in the prevalence of asthma symptoms in children aged 6-7 years and 13-14 years throughout the world with a predominance of atopic conditions in English-speaking countries [9]. Phase two was more focused on selected countries to investigate points of interest that arose from phase 1, whilst phase 3 was a repeat of phase 1 between 2000 and 2003. For the purpose of our study, data from phase 1 in the 13- to 14-year age group was used.
The WHO estimates of mortality and burden of disease for member states for the year 2002 including country specific mortality and burden of disease for over 130 causes were reviewed. These estimates are based on version 3 of the Global Burden of Disease study [10] as published in the World Health Report 2004 [11]. Available data include age-standardised death rates quoted per 100,000 population by cause and member state. Mortality data is grouped into (1) mortality from communicable, maternal, perinatal and nutritional conditions, (2) mortality from noncommunicable diseases, and (3) mortality from injuries. Within the mortality from communicable, maternal, perinatal, and nutritional conditions group, there are specific mortality rates from infectious and parasitic diseases in general as well as that from respiratory infection, tuberculosis and diarrhoeal diseases.
The Alexander project [12] was established in 1992 and is an ongoing surveillance project that examines the susceptibility of bacteria responsible for adult community-acquired respiratory tract infections to a range of antibiotics. Between 1998 and 2002, isolates of Streptococcus pneumonia, Haemophilus influenza, and Moraxella catarrhalis were collected from centres in USA, Africa, Europe, the Far and Middle East, and Latin America and their susceptibility to 23 different antibiotics was tested.
Countries with data both in the ISAAC phase 1 study and in the WHO database were included in the study. For countries with multiple centre data, the mean country prevalence of rhinitis, atopic eczema, rhinoconjunctivitis, and wheezing was taken.
For countries which participated in the ISAAC study, the WHO country-specific age-standardised death rates (quoted per 100,000 inhabitants) from infectious and parasitic diseases were documented. These infections included respiratory infections, tuberculosis and diarrhoeal illness among others. We decided to choose mortality data related to common childhood infections to be able to look at the relationship between these infections and prevalence of common atopic symptoms at a population level for each country.
The Alexander project was used to obtain data for antibiotic susceptibility. Country-specific antibiotic susceptibility data was available for 18 countries which participated in both the Alexander project (between 1998 and 2000) and the ISAAC phase I study. Country-specific antibiotic susceptibility data for S. pneumoniae and H. influenzae were available; these two organisms most commonly cause bacterial infections in childhood. We then proceeded to extract data for the four most important and commonly used antibiotics namely penicillin, erythromycin, doxycycline, and cotrimoxazole. Susceptibility of S. pneumoniae to penicillin and erythromycin was based on National Committee for Clinical Laboratory Standards breakpoints, while susceptibility of S. Pneumoniae to doxycycline and cotrimoxazole and susceptibility of H. influenzae to all antimicrobials were based on pharmacokinetic/pharmacolodynamic breakpoints [12]
Prevalence of rhinitis, atopic eczema, rhinoconjunctivitis, and wheezing and mortality from communicable diseases
Pearson correlation analyses of the country-specific prevalence of rhinitis, atopic eczema, rhinoconjunctivitis, and wheezing to the country-specific mortality from all cause infectious and parasitic diseases, tuberculosis, diarrhoeal illnesses, tropical infections, childhood infections and from respiratory infections were performed.
Prevalence of rhinitis, atopic eczema, rhinoconjunctivitis, and wheezing and antibiotic susceptibility
Pearson correlation analyses were also carried out between the atopic symptoms mentioned above and the country-specific susceptibility of S. Pneumoniae and H. Influenzae to the antibiotics listed earlier.
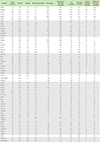
The country-specific prevalence of rhinitis, atopic eczema, rhinoconjunctivitis, and wheezing and mortality from infectious diseases, respiratory infections, tuberculosis, diarrhoeal illnesses, tropical infections, and childhood illnesses are shown in Table 1. There was a significant positive correlation between atopic eczema and mortality from all infectious diseases studied (Fig. 1) (r = 0.33, p = 0.013), diarrhoeal illnesses (r = 0.33, p = 0.014), tropical infections (r = 0.39, p = 0.003), and childhood illnesses (r = 0.35, p = 0.008). There was no significant correlation between the above conditions and the mortality from respiratory infections when these were isolated from other infections (Table 2).
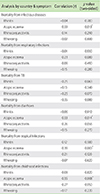
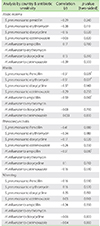
The correlation between prevalence of rhinitis, atopic eczema, rhinoconjunctivitis, and wheezing and antibiotic susceptibility are shown in Table 3.
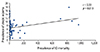
There were significant negative correlations between rhinitis and S. pneumoniae susceptibility to penicillin (r = -0.51, p = 0.029) (Fig. 2) and erythromycin (r = -0.59, p = 0.010) (Fig. 3). There were also significant negative correlations between rhinitis and H. influenzae susceptibility to ampicillin (r = -0.59, p = 0.010) (Fig. 4) and between rhinoconjunctivitis and H. influenzae susceptibility to ampicillin (r = -0.51, p = 0.030) (Fig. 5).
In our ecological study we found a significant positive correlation between the country prevalence of atopic eczema and the country's mortality from infectious diseases. To our knowledge, this has not been previously reported. This relationship was true for total mortality from infectious diseases and parasitic infections (r = 0.33, p = 0.013) as well as to mortality from specific infections including diarrhoea (r = 0.33, p = 0.014), tropical infections (r = 0.39, p = 0.030), and childhood infections (r = 0.35, p = 0.008). There was a similar trend for mortality from respiratory infections but this did not reach statistical significance. Our data suggest that there may be common genetic and/or environmental factors which predispose to both atopic eczema and to the risk and/or severity of infections. These may include factors which predispose to a predilection of a Th2 over a Th1 response, which has been reported to have a genetic element [13]. Th2/Th11 predominance may lead to an increased risk of both atopic and infectious diseases. Atopic eczema is known to be associated with Th2 predominance [1415]. The relation between a Th2 response and increased risk of infections has been demonstrated in animal studies [161718] as well as in humans [1920]. Another factor which may predispose to both atopic and infective conditions in later life could be lack of microbial exposure during early postnatal life, as suggested by the 'hygiene hypothesis' [21]. This may be mediated through alteration of the gut microbiota, which may predispose to atopy [2223] as well as to infections [24].
Alternatively, atopy may itself lead to in Th2/Th1 predominance, which in turn may predispose to an increased infection risk. Atopic disease features increased systemic Th2 responses and Th2/Th1 responses in lesional skin, but there is evidence that allergies also result in impaired innate immunity namely by Th2 polarisation [25]. Many mediator cells in atopy, including mast cells, basophils and eosinophils, are capable of shifting the balance to Th2 polarization through production of interleukin-4 (IL-4) [2627]. IL-4 is also thought to trigger fibronectin production by skin fibroblasts which facilitated bacterial attachment, the first step in establishing an infection [28]. Furthermore, Gros et al. [29] have reported that the enhanced Th2 immunity in patients with atopic dermatitis leads to an impairment of Th1 immune responses by altering the interferon-gamma responses of antigen presenting cells. One can speculate that the positive association between atopy and infectious disease mortality is based on the interaction between interleukins and type 1/2 cytokines. Hoffmann et al. [30] have shown that excessive types 1 and 2 cytokine responses secondary to interleukin regulatory processes in schistosomiasis increases mortality. Upregulation of the Th2 responses in atopic disease in the setting of interleukin deficiency could be one of the possible causes of increased mortality in these patients.
Respiratory infections have been reported to be more common and more severe in individuals with allergic diseases [313233]. Our study, on the other hand, failed to show a correlation of allergic conditions with mortality from respiratory infections at country level, although there was a nonsignificant trend. This is probably due to the fact that such respiratory conditions are usually nonfatal and we studied mortality data rather than incidence of infections. It is therefore probable that total infectious disease mortality in a given country is a better marker of its total infective burden than is mortality from respiratory infections. We also found no association between mortality from tuberculosis and prevalence of atopic eczema. This may be due to the factors other than Th2/Th1 predominance bein g more important in determining tuberculosis mortality, such as coinfection with human immunodeficiency virus.
In contrast to atopic eczema, we found no significant correlation between prevalence of wheezing, rhinoconjunctivitis and rhinitis and infectious disease mortality. This suggests that there are important differences between the pathophysiology of eczema and that of other allergic conditions. These may include the role of staphylococcal superantigen, which has been shown to facilitate epithelial presentation of allergen to Th2 cells [34] and the role of other infective agents in eczema [35].
We also explored the relationship between the same atopic conditions and antibiotic susceptibility of S. pneumoniae and H. influenzae. In our study we found a negative correlation between rhinitis and susceptibility of S. pneumoniae to penicillin and erythromycin and of H. influenzae to ampicillin. There was also a negative correlation between rhinoconjunctivitis and susceptibility of H. influenzae to ampicillin (Fig. 3).
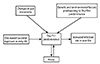
Antibiotic susceptibility is known to be inversely related with local use and abuse of antibiotics. Antibiotic susceptibility depends not only on personal use but also usage in the community as whole, including use in agriculture. It is therefore a strong marker of antibiotic exposure of the community. Our data therefore suggest that increased antibiotic use in a community may be associated with increased risk of atopic disease. Various mechanisms may be implicated. Firstly, increased antibiotic use may decrease bacterial exposure during early postnatal life. Bacterial exposure during these critical periods in early life may help in protecting against atopic disease by decreasing Th1 response [36]. Interestingly, Gram-positive bacteria seem to be more important in stimulating this response [37]. Antibiotic use can also cause long-lasting effects on the constitution and diversity of gut microbiota [3839], which have been linked to increased risk of atopic disease [404142]. Antibiotic use can also affect skin microbiota, which has been implicated in atopic eczema [43]. The proposed interactions between the various inflammatory pathways are summarised in Fig. 6.
This ecological study is based on international research projects involving large numbers of patients of the same age groups. Countries participating in all three studies were utilised for this analysis. The use of age standardized WHO mortality rates and the ISAAC 13- to 14-year-old cohort prevalence rates makes interpretation of results much more accurate and valid.
The main limitation of this study is that the data was obtained from three different research projects which were carried out at different times. However data from the ISAAC study shows there has very little change in prevalence of current wheeze, atopic eczema or rhinoconjunctivitis over time [4445]. Therefore, the time factor is unlikely to have had a major impact on our results. One important limitation of this ecological study is the presence of systematic differences that might have occurred during disease classification, coding and diagnosis in different countries and centers. Another limitation is the potential confounding factors which might influence the distribution and diagnosis of disease in different geographical regions of the world.
The reliability of our findings depends on the reliability of the original data. However all the studies utilised very robust methodology. We also appreciate that we studied only organisms associated with respiratory infections and did not include Staphylococcus aureus which is an important skin pathogen associated with atopic eczema, but this data was not available
We found a significant positive correlation between the prevalence of atopic eczema and mortality from infectious diseases. A high prevalence of eczema could be a marker of environmental and/or genetic predisposition of the population to an enhanced Th2 response, which may lead to an increased infection risk (and hence higher mortality from infectious diseases). One possible environmental factor predisposing to a Th2/Th1 response could be lack of microbial exposure during critical periods in early postnatal life.
We also found a negative correlation between rhinitis and susceptibility of S. pneumoniae to penicillin and erythromycin and of H. influenzae to ampicillin. The overprescription of antibiotics in these allergic conditions could have a direct effect on the susceptibilities of organisms to commonly used antimicrobials. More work is needed to confirm the role of interleukins in infectious disease mortality secondary to atopic disease. Furthermore an in-depth analysis is needed looking at the way antibiotics are prescribed for atopic disease both at a country level and at a global level.
Figures and Tables
 | Fig. 2Correlation of prevalence of rhinitis with Penicillin susceptibility of Streptococcus pneumoniae. |
 | Fig. 3Correlation of prevalence of rhinitis with erythromycin susceptibility of Streptococcus pneumonia. |
 | Fig. 4Correlation of prevalence of rhinitis with ampicillin susceptibility of Haemophilus influenzae. |
 | Fig. 5Correlation of prevalence of rhinoconcjunctivitis with ampicillin susceptibility of Haemophilus influenzae. |
Table 1
Country-specific prevalence of eczema, rhinitis, wheezing and rhinoconjunctivititis in the 13- to 14-year-old age group and age standardized disease mortality
References
1. Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003; 112:6 Suppl. S118–S127.

2. Williams H, Robertson C, Stewart A, Ait-Khaled N, Anabwani G, Anderson R, Asher I, Beasley R, Bjorksten B, Burr M, Clayton T, Crane J, Ellwood P, Keil U, Lai C, Mallol J, Martinez F, Mitchell E, Montefort S, Pearce N, Shah J, Sibbald B, Strachan D, von Mutius E, Weiland SK. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999; 103(1 Pt 1):125–138.

3. Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children's Health. J Invest Dermatol. 2011; 131:67–73.

4. Trepka MJ, Heinrich J, Wichmann HE. The epidemiology of atopic diseases in Germany: an east-west comparison. Rev Environ Health. 1996; 11:119–131.

5. Fsadni P, Fsadni C, Fava S, Montefort S. Correlation of worldwide incidence of type 1 diabetes (DiaMond) with prevalence of asthma and atopic eczema (ISAAC). Clin Respir J. 2012; 6:18–25.

7. Strachan DP. Family size, infection and atopy: the first decade of the "hygiene hypothesis". Thorax. 2000; 55:Suppl 1. S2–S10.

8. Abela AG, Fava S. Association of incidence of type 1 diabetes with mortality from infectious disease and with antibiotic susceptibility at a country level. Acta Diabetol. 2013; 50:859–865.

9. International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee [Internet]. Auckland (NZ): ISAAC;2010. cited 2014 Jan 30. Available from: http://isaac.auckland.ac.nz.
10. Mathers CD, Bernard C, Iburg K, Inoue M, Ma Fat D, Shibuya K, Stein C, Tomijima N, Xu H. Global burden of disease in 2002: data sources, methods and results. Global programme on evidence for health policy discussion paper No. 54 [Internet]. Geneva: World Health Organization;c2015. cited 2014 Jan 30. Available from: http://www.who.int/healthinfo/paper54.pdf.
11. World Health Organization. The World Health Report 2004: changing history [Internet]. Geneva: World Health Organization;c2015. cited 2014 Jan 30. Available from: http://www.who.int/whr/2004/en/.
12. Jacobs MR, Felmingham D, Appelbaum PC, Grüneberg RN. Alexander Project Group. The Alexander Project 1998-2000: susceptibility of pathogens isolated from community-acquired respiratory tract infection to commonly used antimicrobial agents. J Antimicrob Chemother. 2003; 52:229–246.

13. Brown SJ, McLean WH. Eczema genetics: current state of knowledge and future goals. J Invest Dermatol. 2009; 129:543–552.

14. Akdis M, Trautmann A, Klunker S, Daigle I, Kucuksezer UC, Deglmann W, Disch R, Blaser K, Akdis CA. T helper (Th) 2 predominance in atopic diseases is due to preferential apoptosis of circulating memory/effector Th1 cells. FASEB J. 2003; 17:1026–1035.

15. Akkoc T, de Koning PJ, Ruckert B, Barlan I, Akdis M, Akdis CA. Increased activation-induced cell death of high IFN-gamma-producing T(H)1 cells as a mechanism of T(H)2 predominance in atopic diseases. J Allergy Clin Immunol. 2008; 121:652–658.e1.
16. Ehrchen JM, Roth J, Roebrock K, Varga G, Domschke W, Newberry R, Sorg C, Muller-Tidow C, Sunderkotter C, Kucharzik T, Spahn TW. The absence of cutaneous lymph nodes results in a Th2 response and increased susceptibility to Leishmania major infection in mice. Infect Immun. 2008; 76:4241–4250.
17. Fernandez-Cabezudo MJ, Ali SA, Ullah A, Hasan MY, Kosanovic M, Fahim MA, Adem A, al-Ramadi BK. Pronounced susceptibility to infection by Salmonella enterica serovar Typhimurium in mice chronically exposed to lead correlates with a shift to Th2-type immune responses. Toxicol Appl Pharmacol. 2007; 218:215–226.

18. Arendse B, Van Snick J, Brombacher F. IL-9 is a susceptibility factor in Leishmania major infection by promoting detrimental Th2/type 2 responses. J Immunol. 2005; 174:2205–2211.
19. Contoli M, Ito K, Padovani A, Poletti D, Marku B, Edwards MR, Stanciu LA, Gnesini G, Pastore A, Spanevello A, Morelli P, Johnston SL, Caramori G, Papi A. Th2 cytokines impair innate immune responses to rhinovirus in respiratory epithelial cells. Allergy. 2015; 04. 08. [Epub]. DOI: 10.1111/all.12627.

20. Bhattacharya D, Dwivedi VP, Kumar S, Reddy MC, Van Kaer L, Moodley P, Das G. Simultaneous inhibition of T helper 2 and T regulatory cell differentiation by small molecules enhances Bacillus Calmette-Guerin vaccine efficacy against tuberculosis. J Biol Chem. 2014; 289:33404–33411.

21. Schmitz R, Atzpodien K, Schlaud M. Prevalence and risk factors of atopic diseases in German children and adolescents. Pediatr Allergy Immunol. 2012; 23:716–723.

22. Drago L, Toscano M, De Vecchi E, Piconi S, Iemoli E. Changing of fecal flora and clinical effect of L. salivarius LS01 in adults with atopic dermatitis. J Clin Gastroenterol. 2012; 46:Suppl. S56–S63.

23. Iemoli E, Trabattoni D, Parisotto S, Borgonovo L, Toscano M, Rizzardini G, Clerici M, Ricci E, Fusi A, De Vecchi E, Piconi S, Drago L. Probiotics reduce gut microbial translocation and improve adult atopic dermatitis. J Clin Gastroenterol. 2012; 46:Suppl. S33–S40.

24. Endt K, Stecher B, Chaffron S, Slack E, Tchitchek N, Benecke A, Van Maele L, Sirard JC, Mueller AJ, Heikenwalder M, Macpherson AJ, Strugnell R, von Mering C, Hardt WD. The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog. 2010; 6:e1001097.

25. Mrabet-Dahbi S, Maurer M. Does allergy impair innate immunity? Leads and lessons from atopic dermatitis. Allergy. 2010; 65:1351–1356.

26. Werfel T. The role of leukocytes, keratinocytes, and allergen-specific IgE in the development of atopic dermatitis. J Invest Dermatol. 2009; 129:1878–1891.

27. Simon D, Kozlowski E, Simon H. Natural killer T cells expressing IFN-gamma and IL-4 in lesional skin of atopic eczema. Allergy. 2009; 64:1681–1684.
28. Postlethwaite AE, Holness MA, Katai H, Raghow R. Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin 4. J Clin Invest. 1992; 90:1479–1485.

29. Gros E, Petzold S, Maintz L, Bieber T, Novak N. Reduced IFN-γ receptor expression and attenuated IFN-γ response by dendritic cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2011; 128:1015–1021.

30. Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000; 164:6406–6416.

31. Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study. J Allergy Clin Immunol. 2014; 133:1041–1047.
32. Ciprandi G, Tosca MA, Fasce L. Allergic children have more numerous and severe respiratory infections than non-allergic children. Pediatr Allergy Immunol. 2006; 17:389–391.

33. Cirillo I, Marseglia G, Klersy C, Ciprandi G. Allergic patients have more numerous and prolonged respiratory infections than nonallergic subjects. Allergy. 2007; 62:1087–1090.

34. Ardern-Jones MR, Black AP, Bateman EA, Ogg GS. Bacterial superantigen facilitates epithelial presentation of allergen to T helper 2 cells. Proc Natl Acad Sci U S A. 2007; 104:5557–5562.

35. Hussain I, Smith J. Evidence for the transmissibility of atopy: hypothesis. Chest. 2003; 124:1968–1974.
36. Baboonian C, Venables PJ, Williams DG, Williams RO, Maini RN. Cross reaction of antibodies to a glycine/alanine repeat sequence of Epstein-Barr virus nuclear antigen-1 with collagen, cytokeratin, and actin. Ann Rheum Dis. 1991; 50:772–775.

37. Lappalainen MH, Hyvarinen A, Hirvonen MR, Rintala H, Roivainen J, Renz H, Pfefferle PI, Nevalainen A, Roponen M, Pekkanen J. High indoor microbial levels are associated with reduced Th1 cytokine secretion capacity in infancy. Int Arch Allergy Immunol. 2012; 159:194–203.

38. Tanaka S, Kobayashi T, Songjinda P, Tateyama A, Tsubouchi M, Kiyohara C, Shirakawa T, Sonomoto K, Nakayama J. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol Med Microbiol. 2009; 56:80–87.

39. Greenwood C, Morrow AL, Lagomarcino AJ, Altaye M, Taft DH, Yu Z, Newburg DS, Ward DV, Schibler KR. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of Enterobacter. J Pediatr. 2014; 165:23–29.

40. Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012; 129:434–440. 440.e1–440.e2.

41. Forno E, Onderdonk AB, McCracken J, Litonjua AA, Laskey D, Delaney ML, Dubois AM, Gold DR, Ryan LM, Weiss ST, Celedon JC. Diversity of the gut microbiota and eczema in early life. Clin Mol Allergy. 2008; 6:11.

42. Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, Adams H, van Ree R, Stobberingh EE. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007; 56:661–667.

43. Salava A, Lauerma A. Role of the skin microbiome in atopic dermatitis. Clin Transl Allergy. 2014; 4:33.

44. Pearce N, Aït-Khaled N, Beasley R, Mallol J, Keil U, Mitchell E, Robertson C. ISAAC Phase Three Study Group. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2007; 62:758–766.

45. Asher MI, Stewart AW, Wong G, Strachan DP, Garcia-Marcos L, Anderson HR. ISAAC Phase Three Study Group. Changes over time in the relationship between symptoms of asthma, rhinoconjunctivitis and eczema: a global perspective from the International Study of Asthma and Allergies in Childhood (ISAAC). Allergol Immunopathol (Madr). 2012; 40:267–274.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download