Abstract
Asia is one of the most diverse and dynamic continents. Due to recent rapid industrialisation and urbanisation, morbidity patterns are likely to be diverse in Asian populations. Chronic cough is a disease condition resulting from host-environmental interactions, and is associated with a high global epidemiological burden. However, the underlying epidemiology remains unclear, particularly in Asia. We performed a literature search to identify peer-reviewed articles on chronic cough in community-based adult Asian populations that have been published between January 2000 and June 2015. In this review, we aim to examine the epidemiological characteristics and determinants of chronic cough in several geographical areas of Asia.
Asia is one of the largest, most populous, diverse, and dynamic continents. It covers about 30% of the Earth's land area and is home to 60% of the current global population [1]. Economically, Asia includes some of the world's wealthiest as well as some of its poorest countries. The same is also true with regard to education and health status. Over the past several decades, industrialisation and urbanisation have occurred at a rapid pace in Asia, resulting in considerable changes in the health and disease situations in this previously mostly agricultural region. In the context of their unique cultures, lifestyles, environments, and genetic backgrounds, the morbidity patterns within Asian countries are likely to be both diverse and different from those reported elsewhere in the world.
Coughing is an intrinsic defensive response against external inhaled stimuli [2]. The cough reflex is provoked in response to inhalational irritants or infectious stimuli. If the harmful inhalation is repeated, the protective cough response may be seen as chronic (e.g., smoker's cough [3]). However, the characteristics of the cough observed in patients visiting cough clinics are hypersensitive rather than protective responses, which are provoked by trivial stimuli such as cold air, perfume, singing or talking [456]. Except in the context of cough hypersensitivity, these stimuli usually do not elicit a tussive response. It has been suggested that several intrinsic host factors, such as older age, female sex, allergy, or comorbidities, modify host cough responses, leading to chronic hypersensitivity [78].
Considering the role of host-environment interactions in coughing, we hypothesised that chronic cough may have distinct characteristics in Asian countries. However, to our knowledge, there have been no previous studies directly comparing the epidemiological characteristics of the general populations of Asian countries or comparing those of Asia with those of other continents. Our recent meta-analysis indicated that chronic cough was less prevalent in Asia (2-7%) than in Europe (10-15%) and the USA (8-14%) [9]. To examine regional differences, a multinational community-based population survey conforming to a standard protocol is required. Before such a project can be undertaken, it would be useful to have an overview of the characteristics of chronic cough in Asian populations based on a comprehensive review of the relevant literature.
In this review, we examine Asian population-based studies of chronic cough and summarise the epidemiological determinants of chronic cough in Asia.
We performed a semisystematic literature review to identify peer-reviewed articles on chronic cough in community-based adult Asian populations that have been published between January 2000 and June 2015. The PubMed database was searched using the term "chronic cough OR prolonged cough OR persistent cough" for each Asian country. Additional searches were performed in Google Scholar and via cross-referenced articles. For the purpose of analysis, we grouped studies from distinct geographical areas of Asia together.
Several studies have indicated locoregional or nationwide prevalence rates of 2-5% for chronic cough, defined as "cough > 3 months".
In China, Venners et al. [10] compared the prevalence of chronic cough (defined as "cough for 3 or more months of the year") in Beijing, Anqing City, and rural Anqing areas (overall prevalence: 4.5%); they found positive associations between urban areas and chronic cough, but also suggested the potential effects of indoor particulate matter (PM10) exposure. Another urban-rural comparative study in Beijing also demonstrated an association between urban areas and persistent cough [11]. Additionally, a study of 22,528 adults living in rural Beijing areas found that the prevalence of chronic cough, which was 1.9%, was significantly associated with cigarette smoking and insecticide exposure at work as well as marginally associated with fertiliser and wood fuel exposure [12].
In the heavy industry province of Liaoning, northeastern China, chronic cough had a prevalence of 2.3% and was significantly related to smoking (odds ratio [OR], 4.89; 95% confidence interval [CI], 4.04-5.92), passive smoking (OR, 1.32; 95% CI, 1.12-1.55), history of childhood respiratory disease (OR, 3.29; 95% CI, 2.37-4.57), and occupational dust and gas exposure (OR, 1.48; 95% CI, 1.20-1.84 and OR, 1.44; 95% CI, 1.13-1.85, respectively) [13]. Indoor irritant exposure, such as cigarettes at home (OR, 1.63; 95% CI, 1.45-1.84) and heating coal smoke exposure (OR, 1.10; 95% CI, 1.00-1.21), were also reported as risk factors [14]. In Inner Mongolia, chronic arsenic exposure via drinking water was a significant epidemiological issue; compared with unaffected villages, the presence of chronic cough was 13 folds higher among residents living in arsenic-affected areas [15].
A recent online survey in Japan investigating the prevalence of chronic cough in the general population found prevalence rates of cough (defined as "having cough at present") and chronic cough (defined as "having cough lasting ≥ 8 weeks") of 10.2% and 2.2%, respectively [16]. The proportion of those with chronic cough increased with age. About 72.6% of subjects with cough felt that it was troublesome; the main reasons for being troubled by cough were "feeling ashamed to cough in front of other people" (49.0%), "causing trouble to other people" (42.8%), and "having difficulty in conversation" (35.5%). Notably, about 60% of adults with cough had not visited a medical facility for the symptom, suggesting that a considerable proportion of cough patients remain undiagnosed.
Two studies performed in Korea found prevalence rates of chronic cough of 3.5% among middle-aged adults living in the cities Ansan and Ansung (aged 40-69 years) [17] and of 4.6% among the elderly living in Seongnam City (aged ≥65 years) [18]. In the former study [17], the prevalence of chronic cough increased with age and was also significantly associated with undiagnosed airflow obstruction. In the latter study [18], cough among the elderly was investigated in relation to comorbid conditions; the results indicated that chronic cough was significantly related to multiple comorbidities, including asthma, allergic rhinitis, constipation, and poorly controlled diabetes mellitus (glycosylated hemoglobin ≥ 8%). However, chronic cough was not related to other conditions, such as gastroesophageal reflux disease (GERD) and obesity (defined as body mass index ≥ 30 kg/m2), which contrasts with surveys in Western populations [19]. This inconsistency may have been due to differences in prevalence, as obesity and GERD are much less prevalent among adult Koreans (3.8% and 1.1%, respectively [1820]) than among those in the UK (20.6% and 14.6-22.9%, respectively [19]).
A nationwide survey in Taiwan reported that the prevalence of chronic cough with phlegm (3.1%) was positively related to job stress [21]. This may be due to the positive association between stress and GERD. In a case-control study involving 109 temple workers and 118 controls, chronic cough was found to be 23 folds more frequent among temple workers who were frequently exposed to airborne pollutants emitted by burning incense [22].
Several studies have examined the relationships between chronic cough and environmental pollutant exposure as well as with Asian dietary habits. In developing countries, there is significant concern over chronic cough as a sign of respiratory tract infection, such as pulmonary tuberculosis (TB) or paragonimiasis. In particular, persistent cough for longer than 2 or 3 weeks has been considered an indicator of suspected TB in several Southeast Asian countries [23242526]. In these countries, national guidelines suggest that patients with persistent cough have their sputum examined for acid-fast bacilli.
A survey in Laos using a representative sample found that 5.1% of 374 chronic cough patients (cough > 3 weeks) had pulmonary TB [27]. It is also noteworthy that paragonimiasis, which is endemic in the area, was detected in 7.0% of chronic cough patients [27]. The endemicity of TB and paragonimiasis was also problematic in the province of Zamboanga del Norte, the Philippines (prevalences of 1.9% and 6.7%, respectively) among subjects with cough > 2 weeks in duration [28]. In northern areas of Vietnam, paragonimiasis is endemic, probably due to the dietary habit of eating raw crab; one report indicated that chronic cough was present in all cases of paragonimiasis detected during 1994-2000 (n = 178) [29].
In Indonesia, persistent cough (defined as "cough > 3 months of the year") among 16,663 pairs of junior high school students and their mothers living in provincial capital cities was positively associated with living near a major road and active smoking [30].
The Singapore Chinese Health Study provided novel insights into the epidemiology of chronic cough with phlegm in the middle-aged adult population in Singapore. The most notable findings were that dietary patterns contributed significantly to the incidence of chronic cough with phlegm. First, in their longitudinal follow-up survey, high-fibre diets were inversely correlated with new-onset chronic cough with phlegm, with OR of 0.61 (95% CI, 0.43-0.82) for daily intake of nonstarch polysaccharide, a major component of dietary fibre (comparing the fourth and first quartiles) [31]. Second, in their later study, the "meat-dim sum" dietary pattern (characterised by pork and chicken dim sum foods and noodle dishes) was positively associated with new-onset cough with phlegm (OR, 1.43; 95% CI, 1.08-1.89; comparing fourth and first quartiles), even after adjustment for nonstarch polysaccharide intake [32]. In addition, this study also indicated that living with a smoker during childhood was related to having chronic dry cough in adulthood among a cohort of middle-aged never-smokers [33]. Occupational vapour exposure, such as chemical solvents or cooking oils, was also positively associated with chronic dry cough [34].
In Thailand, the prevalence of persistent cough with phlegm and that of frequent cough (> 4 times/day) among the traffic police in Bangkok (high PM10 level area) were 2.6% and 12.8%, respectively. However, similar symptoms were reported in only 1.5% and 8.8%, respectively, of general police officers in Ayutthaya (low PM10 level area) [35]. The effects of occupational risk factors were also documented in several studies. Glass microfibre factory workers had double the risk of recurrent/prolonged cough compared with office workers [36]. Workers at a rubber tree furniture factory had higher risk of cough, particularly if they were exposed to ethyl cyanoacrylate glue, compared with office workers [37]. The prevalence of chronic cough in roofing fibre cement workers exposed to high respiratory dust levels was higher than that of unexposed subjects (OR, 1.34; 95% CI, 0.80-2.25), although the difference was not statistically significant [38].
The prevalences of longstanding cough (defined as a positive response to "Have you had a longstanding cough over the past several years?") in urban and rural areas of Vietnam were compared, and the results indicated a higher prevalence in the rural area, Bavi, than in the urban area, Hanoi (18.1% and 12.0%, respectively, p < 0.001). However, in both areas, current smoking was a significant risk factor for longstanding cough (vs. nonsmokers: OR, 1.45; 95% CI, 1.16-1.80) [39].
In South Asia, the prevalence data were obtained primarily from India. In terms of risk factors, environmental and occupational respiratory effects were examined in several countries. However, similar to Southeast Asia, there was also concern over TB in cases with persistent cough, > 2-3 weeks, in some areas [4041].
The effects of exposure to arsenic in drinking water have been studied in detail in Bangladesh. In a large-scale prospective cohort study, the hazard ratio of developing chronic cough was positively correlated with water arsenic and urinary arsenic levels [42]. Indoor use of unprocessed biomass fuel (e.g., from wood or crop residues) was found to be a risk factor for chronic bronchitis [43]. In a rural population, sputum smear-positive TB was found in 1.4% of adults with cough > 3 weeks [40].
In India, Chhabra et al. [44] conducted a cross-sectional study of 3,465 subjects in an urban area of East Delhi. The prevalence of chronic cough, as defined by the Medical Research Council definition of chronic bronchitis, was 2%. In a similar study conducted in South India, the prevalence of cough in 4,333 adults (age > 30 years) was 2.5% [45]. In both surveys, older age and smoking were demonstrated to be independent risk factors for chronic cough [4445]. Higher prevalence rates, 5.3% and 7.3%, were reported by Kumar et al. [46] in samples of 908 and 1,105 individuals from an industrial and a nonindustrial town in North India, respectively. In a recent study, the prevalence of chronic cough according to the definition of chronic bronchitis was reported to be 2.7% among women from a rural area of Central India [47].
Although not addressing the prevalence of chronic cough itself, large population-based multicentre reports from India have addressed the prevalence of chronic obstructive pulmonary disease (COPD) using the standard criteria for chronic bronchitis (cough with expectoration for more than 3 months in a year over the past 2 or more years). In a cross-sectional study across four divergent geographical areas, COPD was diagnosed in 4.1% of a total of 35,295 subjects [48]. The same group also reported the prevalence of respiratory symptoms and risk factors for asthma and chronic bronchitis in 85,105 men and 84,470 women from 12 urban and 11 rural sites across India. The prevalence of chronic bronchitis was reported to 3.5%, and large differences in prevalence were seen between the different centres based on age, sex, and place of residence. Cough at night, in the morning and phlegm in the morning were also reported in this study. The prevalence (range) of these symptoms was 4.6% (1.6% to 14.5%), 4.3% (1.6% to 11.5%) and 3.8% (0.9% to 11.5%) respectively [49].
The population in India is growing rapidly, and India is projected to become the world's most populous nation by the middle of this century. Both industrialisation and urban growth have occurred at a rapid pace in this previously agricultural society, leading to different and diverse risk factors for respiratory morbidity. Exposure to biomass fuels is very high in the agrarian setting, and industrial air pollution is a major factor in urban areas with industry. The use of biomass fuel has long been recognised as a risk factor for respiratory morbidity [50]. The indoor use of solid and biomass fuel is common in India as well as in other developing areas of the world. This household air pollution secondary to the indoor combustion of solid fuel is associated with respiratory morbidity and is a well-recognised factor associated with the development of COPD [5152]. A minimum threshold of biomass fuel exposure has been suggested as a significant risk factor for the development of chronic bronchitis [53]. This association between respiratory symptoms and exposure to indoor pollution was also observed in the previously mentioned large population-based survey [49].
An association between outdoor air pollution due to industry and respiratory symptoms has been demonstrated [46]. Outdoor air pollution in the rapidly expanding metropolises of India is a significant issue and is thought to be a cause of significant respiratory morbidity [54]. Clearly, this is a phenomenon not limited to India, with the "Beijing Cough" being as notorious as the "Delhi Smog". However, this air pollution will have different effects according to the local milieu. For example, respiratory morbidity in traffic policemen has been attributed to pollution from vehicle exhaust fumes because these traffic policemen work in traffic and are directly exposed to such fumes [55].
A "beedi" is a form of a cigarette in which tobacco is wrapped in a tendu leaf, tied with a string at one end, and smoked like a standard cigarette. As these are inexpensive compared with cigarettes, they are very popular, and they are used more commonly than conventional cigarettes in India. Conventional cigarettes are popular in more affluent population groups, whereas beedis are popular among those in the lower socioeconomic groups. The harmful effects of smoking beedis are thought to be greater than those of traditional cigarettes, and smoking beedis is a well-recognised risk factor for respiratory symptoms. Respiratory symptoms, including cough, are more commonly reported by beedi smokers than by cigarette smokers [56]. In fact, significant respiratory morbidity has also been reported in beedi binders, (i.e., those who roll the leaf with the tobacco inside and bind it) [57].
A few studies in Pakistan and Sri Lanka have examined chronic cough resulting from specific occupations, such as those involving brick kilns, textile work, or gem cutting [585960]. Additionally, 15.8% of subjects in a periurban area of Karachi City, Pakistan with cough lasting ≥ 2 weeks were found to have pulmonary TB [41].
In the Middle East, the prevalence of chronic bronchitis (defined as "the presence of cough or sputum production for at least 3 months in each of 2 consecutive years") has been reported as 0.6-3.4% [61]. In Turkey, the prevalence of chronic cough (>3 months) was reported as 10.6% among adults living in Samsun, North Anatolia and was significantly related to older age and smoking [62].
Mustard gas, which affects the skin, eyes, and lungs, was used as a chemical warfare agent in the Iran-Iraq war [63]. Many patients in this area are thought to have chronic cough related to previous mustard gas exposure in the absence of smoking history [64]. Meanwhile, in the United Arab Emirates, occupational cement dust exposure was found to be a risk factor for chronic cough [6566]. In Jordan, a community-population survey reported that the prevalence of persistent cough for longer than 3 weeks (defined as suspected TB) was 2.5%, and smear-positive TB was present in only 0.1% of subjects with cough [67].
Based on the studies discussed above, we schematically summarised the epidemiological determinants of chronic cough in Asia in Fig. 1. First, exposure to environmental pollutants and irritants was a major determinant and was particularly relevant in rapidly developing countries. These irritants and pollutants may provoke acute cough responses with a protective function and also induce chronic cough responses with chronic exposure, which is analogous to chronic bronchitis as a response to tobacco smoking.
Second, infection, such as pulmonary TB or paragonimiasis, continues to be an important risk factor for persistent cough (>2-3 weeks) in some Asian populations. The frequencies of these infectious conditions among patients with chronic cough >2-3 months remain to be elucidated. Third, previous regional incidents, such as chronic arsenic or chemical warfare exposure, also appeared to have affected respiratory morbidities, including chronic cough. In addition, an Asian dietary pattern may also be a modifier of the incidence of chronic cough, although further studies are required to determine the mechanisms underlying these effects.
Although we did not review recent epidemiological reports related to angiotensin converting enzyme inhibitor (ACEi) therapy, cough resulting from ACEi is a relatively well-recognised issue, particularly in Asians. In a previous comparative study conducted in the 1990s, Chinese subjects had a much higher risk of ACEi-related cough than Caucasians [68]. In a meta-analysis, the incidence of cough due to ACEi has been also reported to be 2.7 folds higher in East Asian populations compared with Caucasian ones [69].
Age, gender, and comorbidities are other important factors. The predominance of older females is a typical demographic characteristic of chronic cough patients at cough clinics internationally [8]. Female sex is thought to be an intrinsic risk factor for developing chronic cough; both females reporting chronic cough and healthy female volunteers have more sensitive cough responses to tussigen inhalation [470]. Allergic comorbidities are also important in chronic cough, as allergic inflammation may lead to afferent neuronal hypersensitivity [71]; significant associations among cough, asthma, and allergic rhinitis have been consistently found in various general population surveys, including those in Asia and Europe [187273]. However, considering the rapid westernisation and aging trends in Asia, the comorbidity associations of chronic cough may change and become more complex in the near future.
In this review, we examined the recent Asian literature and summarised several issues related to chronic cough in Asian countries. As summarised in Fig. 1, several distinct factors seemed to underlie the epidemiology of chronic cough in Asian countries. Potentially serious infection was relevant, especially in developing countries. Exposure to pollutants, which is specific to urban and industrialised areas compared with rural and agrarian ones, was a common theme across Asian countries. Although cough clearly has some universally applicable determinants, it is necessary to consider these unique attributes of chronic cough in Asia.
Chronic cough is a significant health issue with high prevalence [9], but the epidemiology has attracted little attention, as it has been mostly considered to be just one of the symptoms resulting from other pulmonary or nonpulmonary conditions [74]. However, as a result of recent advances in mechanistic studies, chronic cough has begun to be understood as a clinical entity with an intrinsic pathophysiology [6]. To gain a better understanding of chronic cough, further epidemiological investigations are required. We propose that methodological frameworks should be prepared and standardised to enable multinational surveys to investigate the epidemiology of chronic cough itself.
Figures and Tables
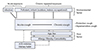 | Fig. 1Schematic representation of epidemiological determinants of chronic cough in Asia based on findings from general adult population surveys. Exposure to environmental pollutants and irritants is a risk factor for acute and chronic cough, which may be particularly important in rapidly developing countries. Infection, such as pulmonary tuberculosis or paragonimiasis, is a frequent cause of chronic cough in some areas. Host factors, such as older age, female sex, allergy, or comorbidities, may also predispose individuals to chronic cough hypersensitivity. Angiotensin converting enzyme (ACE) inhibitor-induced cough is more frequent in Asian than in Caucasian populations. Dietary habits may also influence the incidence of chronic cough. |
References
1. Wikipedia. Asia [Internet]. San Francisco: Wikipedia;c2015. cited 2015 Jun 15. Available from: http://en.wikipedia.org/wiki/Asia.
3. Morice AH, Fontana GA, Sovijarvi AR, Pistolesi M, Chung KF, Widdicombe J, O'Connell F, Geppetti P, Gronke L, De Jongste J, Belvisi M, Dicpinigaitis P, Fischer A, McGarvey L, Fokkens WJ, Kastelik J. ERS Task Force. The diagnosis and management of chronic cough. Eur Respir J. 2004; 24:481–492.
4. Song WJ, Kim JY, Jo EJ, Lee SE, Kim MH, Yang MS, Kang HR, Park HW, Chang YS, Min KU, Cho SH. Capsaicin cough sensitivity is related to the older female predominant feature in chronic cough patients. Allergy Asthma Immunol Res. 2014; 6:401–408.

5. Matsumoto H, Tabuena RP, Niimi A, Inoue H, Ito I, Yamaguchi M, Otsuka K, Takeda T, Oguma T, Nakaji H, Tajiri T, Iwata T, Nagasaki T, Jinnai M, Matsuoka H, Mishima M. Cough triggers and their pathophysiology in patients with prolonged or chronic cough. Allergol Int. 2012; 61:123–132.

6. Morice AH, Millqvist E, Belvisi MG, Bieksiene K, Birring SS, Chung KF, Dal Negro RW, Dicpinigaitis P, Kantar A, McGarvey LP, Pacheco A, Sakalauskas R, Smith JA. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J. 2014; 44:1132–1148.

7. Song WJ, Chang YS, Morice AH. Changing the paradigm for cough: does 'cough hypersensitivity' aid our understanding? Asia Pac Allergy. 2014; 4:3–13.

8. Morice AH, Jakes AD, Faruqi S, Birring SS, McGarvey L, Canning B, Smith JA, Parker SM, Chung KF, Lai K, Pavord ID, van den Berg J, Song WJ, Millqvist E, Farrell MJ, Mazzone SB, Dicpinigaitis P. Chronic Cough Registry. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur Respir J. 2014; 44:1149–1155.

9. Song WJ, Chang YS, Faruqi S, Kim JY, Kang MG, Kim S, Jo EJ, Kim MH, Plevkova J, Park HW, Cho SH, Morice AH. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J. 2015; 45:1479–1481.

10. Venners SA, Wang B, Ni J, Jin Y, Yang J, Fang Z, Xu X. Indoor air pollution and respiratory health in urban and rural China. Int J Occup Environ Health. 2001; 7:173–181.

11. Wang X, Deng FR, Lv HB, Wu SW, Guo XB. Long-term effects of air pollution on the occurrence of respiratory symptoms in adults of Beijing. Beijing Da Xue Xue Bao. 2011; 43:356–359.
12. Zhang LX, Enarson DA, He GX, Li B, Chan-Yeung M. Occupational and environmental risk factors for respiratory symptoms in rural Beijing, China. Eur Respir J. 2002; 20:1525–1531.

13. Wilson D, Takahashi K, Pan G, Chan CC, Zhang S, Feng Y, Hoshuyama T, Chuang KJ, Lin RT, Hwang JS. Respiratory symptoms among residents of a heavy-industry province in China: prevalence and risk factors. Respir Med. 2008; 102:1536–1544.

14. Qian Z, He Q, Kong L, Xu F, Wei F, Chapman RS, Chen W, Edwards RD, Bascom R. Respiratory responses to diverse indoor combustion air pollution sources. Indoor Air. 2007; 17:135–142.

15. Guo X, Fujino Y, Chai J, Wu K, Xia Y, Li Y, Lv J, Sun Z, Yoshimura T. The prevalence of subjective symptoms after exposure to arsenic in drinking water in Inner Mongolia, China. J Epidemiol. 2003; 13:211–215.

16. Fujimura M. Frequency of persistent cough and trends in seeking medical care and treatment-results of an internet survey. Allergol Int. 2012; 61:573–581.

17. Shin C, Lee S, Abbott RD, Kim JH, Lee SY, In KH, Kimm K. Respiratory symptoms and undiagnosed airflow obstruction in middle-aged adults: the Korean Health and Genome Study. Chest. 2004; 126:1234–1240.
18. Song WJ, Morice AH, Kim MH, Lee SE, Jo EJ, Lee SM, Han JW, Kim TH, Kim SH, Jang HC, Kim KW, Cho SH, Min KU, Chang YS. Cough in the elderly population: relationships with multiple comorbidity. PLoS One. 2013; 8:e78081.

19. Ford AC, Forman D, Moayyedi P, Morice AH. Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax. 2006; 61:975–979.

20. Song WJ, Kim SH, Lim S, Park YJ, Kim MH, Lee SM, Lee SB, Kim KW, Jang HC, Cho SH, Min KU, Chang YS. Association between obesity and asthma in the elderly population: potential roles of abdominal subcutaneous adiposity and sarcopenia. Ann Allergy Asthma Immunol. 2012; 109:243–248.

21. Cheng Y, Guo YL, Yeh WY. A national survey of psychosocial job stressors and their implications for health among working people in Taiwan. Int Arch Occup Environ Health. 2001; 74:495–504.

22. Ho CK, Tseng WR, Yang CY. Adverse respiratory and irritant health effects in temple workers in Taiwan. J Toxicol Environ Health A. 2005; 68:1465–1470.

23. Wahyuni CU, Budiono , Rahariyani LD, Sulistyowati M, Rachmawati T, Djuwari , Yuliwati S, van der Werf MJ. Obstacles for optimal tuberculosis case detection in primary health centers (PHC) in Sidoarjo district, East Java, Indonesia. BMC Health Serv Res. 2007; 7:135.

24. Ahmad RA, Richardus JH, de Vlas SJ. Care-seeking behaviour among individuals with TB symptoms in Jogjakarta Province, Indonesia: a community-based study. Int Health. 2013; 5:51–57.

25. Hoa NP, Thorson AE, Long NH, Diwan VK. Knowledge of tuberculosis and associated health-seeking behaviour among rural Vietnamese adults with a cough for at least three weeks. Scand J Public Health Suppl. 2003; 62:59–65.
26. Hoa NB, Tiemersma EW, Sy DN, Nhung NV, Vree M, Borgdorff MW, Cobelens FG. Health-seeking behaviour among adults with prolonged cough in Vietnam. Trop Med Int Health. 2011; 16:1260–1267.

27. Odermatt P, Nanthaphone S, Barennes H, Chanthavysouk K, Tran DS, Kosanouvong B, Keola S, Mathouchanh P, Choumlivong K, Keoluangkhot V, Phoumindr N, Nanthanavone S, Phrommala S, Degrémont A, Strobel M. Improving tuberculosis case detection rate with a lay informant questionnaire: an experience from the Lao People's Democratic Republic. Bull World Health Organ. 2007; 85:727–731.

28. Belizario V Jr, Totanes FI, Asuncion CA, De Leon W, Jorge M, Ang C, Naig JR. Integrated surveillance of pulmonary tuberculosis and paragonimiasis in Zamboanga del Norte, the Philippines. Pathog Glob Health. 2014; 108:95–102.

29. Van De N. Epidemiology, pathology and treatment of paragonimiasis in Vietnam. Southeast Asian J Trop Med Public Health. 2004; 35:Suppl 1. 331–336.
30. Duki MI, Sudarmadi S, Suzuki S, Kawada T, Tri-Tugaswati A. Effect of air pollution on respiratory health in Indonesia and its economic cost. Arch Environ Health. 2003; 58:135–143.

31. Butler LM, Koh WP, Lee HP, Yu MC, London SJ. Dietary fiber and reduced cough with phlegm: a cohort study in Singapore. Am J Respir Crit Care Med. 2004; 170:279–287.
32. Butler LM, Koh WP, Lee HP, Tseng M, Yu MC, London SJ. Singapore Chinese Health Study. Prospective study of dietary patterns and persistent cough with phlegm among Chinese Singaporeans. Am J Respir Crit Care Med. 2006; 173:264–270.

33. David GL, Koh WP, Lee HP, Yu MC, London SJ. Childhood exposure to environmental tobacco smoke and chronic respiratory symptoms in non-smoking adults: the Singapore Chinese Health Study. Thorax. 2005; 60:1052–1058.

34. LeVan TD, Koh WP, Lee HP, Koh D, Yu MC, London SJ. Vapor, dust, and smoke exposure in relation to adult-onset asthma and chronic respiratory symptoms: the Singapore Chinese Health Study. Am J Epidemiol. 2006; 163:1118–1128.
35. Karita K, Yano E, Jinsart W, Boudoung D, Tamura K. Respiratory symptoms and pulmonary function among traffic police in Bangkok, Thailand. Arch Environ Health. 2001; 56:467–470.

36. Sripaiboonkij P, Sripaiboonkij N, Phanprasit W, Jaakkola MS. Respiratory and skin health among glass microfiber production workers: a cross-sectional study. Environ Health. 2009; 8:36.

37. Sripaiboonkij P, Phanprasit W, Jaakkola MS. Respiratory and skin effects of exposure to wood dust from the rubber tree Hevea brasiliensis. Occup Environ Med. 2009; 66:442–447.

38. Thepaksorn P, Pongpanich S, Siriwong W, Chapman RS, Taneepanichskul S. Respiratory symptoms and patterns of pulmonary dysfunction among roofing fiber cement workers in the south of Thailand. J Occup Health. 2013; 55:21–28.

39. Lam HT, Ronmark E, Tuong NV, Ekerljung L, Chuc NT, Lundback B. Increase in asthma and a high prevalence of bronchitis: results from a population study among adults in urban and rural Vietnam. Respir Med. 2011; 105:177–185.
40. Zaman K, Yunus M, Arifeen SE, Baqui AH, Sack DA, Hossain S, Rahim Z, Ali M, Banu S, Islam MA, Begum N, Begum V, Breiman RF, Black RE. Prevalence of sputum smear-positive tuberculosis in a rural area in Bangladesh. Epidemiol Infect. 2006; 134:1052–1059.

41. Akhtar S, White F, Hasan R, Rozi S, Younus M, Ahmed F, Husain S, Khan BS. Hyperendemic pulmonary tuberculosis in peri-urban areas of Karachi, Pakistan. BMC Public Health. 2007; 7:70.

42. Parvez F, Chen Y, Brandt-Rauf PW, Slavkovich V, Islam T, Ahmed A, Argos M, Hassan R, Yunus M, Haque SE, Balac O, Graziano JH, Ahsan H. A prospective study of respiratory symptoms associated with chronic arsenic exposure in Bangladesh: findings from the Health Effects of Arsenic Longitudinal Study (HEALS). Thorax. 2010; 65:528–533.

43. Alim MA, Sarker MA, Selim S, Karim MR, Yoshida Y, Hamajima N. Respiratory involvements among women exposed to the smoke of traditional biomass fuel and gas fuel in a district of Bangladesh. Environ Health Prev Med. 2014; 19:126–134.

44. Chhabra P, Sharma G, Kannan AT. Prevalence of respiratory disease and associated factors in an urban area of delhi. Indian J Community Med. 2008; 33:229–232.

45. Mahesh PA, Jayaraj BS, Prabhakar AK, Chaya SK, Vijayasimha R. Prevalence of chronic cough, chronic phlegm & associated factors in Mysore, Karnataka, India. Indian J Med Res. 2011; 134:91–100.
46. Kumar R, Sharma M, Srivastva A, Thakur JS, Jindal SK, Parwana HK. Association of outdoor air pollution with chronic respiratory morbidity in an industrial town in northern India. Arch Environ Health. 2004; 59:471–477.

47. Dutta S, Deshmukh PR. Prevalence and determinants of self-reported chronic bronchitis among women in rural Central India. Med J Armed Forces India. 2015; 71:48–52.

48. Jindal SK, Aggarwal AN, Chaudhry K, Chhabra SK, D'Souza GA, Gupta D, Katiyar SK, Kumar R, Shah B, Vijayan VK. Asthma Epidemiology Study Group. A multicentric study on epidemiology of chronic obstructive pulmonary disease and its relationship with tobacco smoking and environmental tobacco smoke exposure. Indian J Chest Dis Allied Sci. 2006; 48:23–29.
49. Jindal SK, Aggarwal AN, Gupta D, Agarwal R, Kumar R, Kaur T, Chaudhry K, Shah B. Indian study on epidemiology of asthma, respiratory symptoms and chronic bronchitis in adults (INSEARCH). Int J Tuberc Lung Dis. 2012; 16:1270–1277.

50. Behera D, Jindal SK. Respiratory symptoms in Indian women using domestic cooking fuels. Chest. 1991; 100:385–388.

51. Prasad R, Singh A, Garg R, Giridhar GB. Biomass fuel exposure and respiratory diseases in India. Biosci Trends. 2012; 6:219–228.

52. Sood A. Indoor fuel exposure and the lung in both developing and developed countries: an update. Clin Chest Med. 2012; 33:649–665.
53. Mahesh PA, Jayaraj BS, Prabhakar AK, Chaya SK, Vijaysimha R. Identification of a threshold for biomass exposure index for chronic bronchitis in rural women of Mysore district, Karnataka, India. Indian J Med Res. 2013; 137:87–94.
54. Chowgule RV, Shetye VM, Parmar JR, Bhosale AM, Khandagale MR, Phalnitkar SV, Gupta PC. Prevalence of respiratory symptoms, bronchial hyperreactivity, and asthma in a megacity. Results of the European community respiratory health survey in Mumbai (Bombay). Am J Respir Crit Care Med. 1998; 158:547–554.
55. Singh V, Sharma BB, Yadav R, Meena P. Respiratory morbidity attributed to auto-exhaust pollution in traffic policemen of Jaipur, India. J Asthma. 2009; 46:118–121.

56. Chhabra SK, Rajpal S, Gupta R. Patterns of smoking in Delhi and comparison of chronic respiratory morbidity among beedi and cigarette smokers. Indian J Chest Dis Allied Sci. 2001; 43:19–26.
57. Chattopadhyay BP, Gangopadhyay PK, Das A, Alam SJ, Hossain M, Chowdhury A, Kundu A. Respiratory response to tobacco dust exposure among biddi binders: A follow up and bronchodilator study. Indian J Occup Environ Med. 2014; 18:57–63.

58. Shaikh S, Nafees AA, Khetpal V, Jamali AA, Arain AM, Yousuf A. Respiratory symptoms and illnesses among brick kiln workers: a cross sectional study from rural districts of Pakistan. BMC Public Health. 2012; 12:999.

59. Nafees AA, Fatmi Z, Kadir MM, Sathiakumar N. Pattern and predictors for respiratory illnesses and symptoms and lung function among textile workers in Karachi, Pakistan. Occup Environ Med. 2013; 70:99–107.

60. Prathapan S, Hettiarachchi P, Wimalasekara SW. Respiratory illnesses and ventilatory function among gem cutters in Sri Lanka. Ceylon Med J. 2013; 58:29–31.

61. Tageldin MA, Nafti S, Khan JA, Nejjari C, Beji M, Mahboub B, Obeidat NM, Uzaslan E, Sayiner A, Wali S, Rashid N, El Hasnaoui A. BREATHE Study Group. Distribution of COPD-related symptoms in the Middle East and North Africa: results of the BREATHE study. Respir Med. 2012; 106:Suppl 2. S25–S32.

62. Hamzacebi H, Unsal M, Kayhan S, Bilgin S, Ercan S. Prevalence of asthma and respiratory symptoms by age, gender and smoking behaviour in Samsun, North Anatolia Turkey. Tuberk Toraks. 2006; 54:322–329.
63. Razavi SM, Ghanei M, Salamati P, Safiabadi M. Long-term effects of mustard gas on respiratory system of Iranian veterans after Iraq-Iran war: a review. Chin J Traumatol. 2013; 16:163–168.
64. Ghanei M, Hosseini AR, Arabbaferani Z, Shahkarami E. Evaluation of chronic cough in chemical chronic bronchitis patients. Environ Toxicol Pharmacol. 2005; 20:6–10.

65. Ahmed HO, Abdullah AA. Dust exposure and respiratory symptoms among cement factory workers in the United Arab Emirates. Ind Health. 2012; 50:214–222.

66. Al-Neaimi YI, Gomes J, Lloyd OL. Respiratory illnesses and ventilatory function among workers at a cement factory in a rapidly developing country. Occup Med (Lond). 2001; 51:367–373.

67. Rumman KA, Sabra NA, Bakri F, Seita A, Bassili A. Prevalence of tuberculosis suspects and their healthcare-seeking behavior in urban and rural Jordan. Am J Trop Med Hyg. 2008; 79:545–551.

68. Woo KS, Norris RM, Nicholls G. Racial difference in incidence of cough with angiotensin-converting enzyme inhibitors (a tale of two cities). Am J Cardiol. 1995; 75:967–968.
69. McDowell SE, Coleman JJ, Ferner RE. Systematic review and meta-analysis of ethnic differences in risks of adverse reactions to drugs used in cardiovascular medicine. BMJ. 2006; 332:1177–1181.

70. Fujimura M, Kasahara K, Kamio Y, Naruse M, Hashimoto T, Matsuda T. Female gender as a determinant of cough threshold to inhaled capsaicin. Eur Respir J. 1996; 9:1624–1626.

71. Song WJ, Chang YS. Cough hypersensitivity as a neuro-immune interaction. Clin Transl Allergy. 2015; 5:24.

72. Plevkova J, Song WJ. Chronic cough in subjects with upper airway diseases: analysis of mechanisms and clinical applications. Asia Pac Allergy. 2013; 3:127–135.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download