Abstract
Background
Accumulating data indicates that pholcodine (PHO)-consuming countries have higher sero-prevalences of immunoglobulin E (IgE)-antibodies to PHO and suxamethonium (SUX) and increased frequencies of IgE-mediated anaphylaxis to neuromuscular blocking agents (NMBAs) than nonconsuming. Withdrawing PHO-containing cough syrups resulted in a significant decrease of cases with anaphylaxis in Scandinavia. Nevertheless, the European Medicines Agency in 2011 advised to continue the unrestricted use throughout the European Union.
Objective
To extend studies on PHO consumption and prevalence of IgE-sensitization to morphine (MOR), PHO, and SUX to countries representing high (Australia), and low (Korea and Japan), consumers, respectively.
Methods
IgE-antibodies to SUX, MOR, and PHO in atopic subjects were determined by immunoassay and compared with official figures for PHO consumption and reported anaphylaxis to NMBA.
Results
The prevalences of IgE-antibodies to PHO, MOR, and SUX were 10%, 8.6%, and 4.3%, respectively, in Australia. The corresponding figures for Japan were 0.8%, 0.8%, and 1.5%, and for Korea 1.0% to PHO and 0.5% to MOR and SUX. Of the SUX-positive sera, 100% were positive to PHO or MOR in Australia and 0% in Japan and Korea.
During the past decade, an increasing amount of data has accumulated indicating that pholcodine (PHO) consuming countries have higher serological prevalences of immunoglobulin E (IgE)-antibodies to PHO, and suxamethonium (SUX), than nonconsuming [1]. Major PHO consuming countries, like Australia, France, United Kingdom and, until recently, Norway, reported high frequencies of IgE-mediated anaphylactic reactions to neuromuscular blocking agents (NMBAs) used during general anesthesia. Since a large number of the reactions occur in patients not previously exposed to NMBAs, an NMBA-specific IgE-sensitization must have taken place before the adverse event. The PHO molecule, which shares the major allergenic epitope, the quaternary ammonium ion, quaternary ammonium ion (QAI), with NMBA [2], has been shown to be very immunogenic, possibly sensitizing as many as 20-25% of individuals exposed [3], in addition to having an enormous polyclonal boosting capacity, increasing IgE levels up to 100 folds in sensitized and re-exposed individuals [4, 5]. Thus, an NMBA-related presensitization may be induced by exposure to the widespread and unrestricted use of PHO-containing cough and cold medicines sold over the counter in around a third of the nations in the world [1]. The European Medicines Agency (EMA) in 2011, after a formal review of all available data, however, advised to continue the unrestricted use of PHO-containing cough medicines throughout the European Union [6].
Among the Scandinavian countries, Denmark has never had PHO-containing drugs, while Sweden, Norway, and Finland withdrew all PHO-drugs from their markets in 1985, 2007, and 2010, respectively. Thus, IgE-sensitized individuals, as well as IgE-mediated anaphylactic reactions to NMBAs, have become true rarities in Scandinavia [3].
In Australia the problem with anaphylaxis related to NMBAs has been under observation for many years [7]. From official sources we have obtained information that cough syrups containing PHO are commonly used in the country, whereas Japan and Korea are nonconsuming countries (www.incb.org.).
The aim of this study was to further study the possible link between PHO consumption and IgE-sensitization to QAI.
Serum was collected at the collaborating centres either as superfluous volumes from routine allergy laboratories (Japan and Korea), or were drawn from patients referred for allergological examinations at a referral allergy centre (Australia). After obtaining informed consent, de-identified sera were collected during the years of 2009-2012 and stored at the respective centres at -20℃ until analysed. Inclusion criteria were available serum volumes of at least 1.0 mL, a positive Phadiatop test (Thermo Fisher Scientific, Uppsala, Sweden) or skin prick test to a panel of common inhalant allergens and an IgE level below 10,000 kU/L (ImmunoCAP, Thermo Fisher Scientific) or a Phadiatop below 120 kUA/L if no IgE-value was available. The study was approved by the local Ethics committees for medical research at each participating centre.
Each serum sample was tested for IgE (kU/L), IgE-antibodies (kUA/L) to a mix of common inhalant allergens, Phadiatop, and for IgE-antibodies to PHO (c261), morphine (MOR) (c260), and SUX (c202) by ImmunoCAP Specific IgE (Thermo Fisher Scientific), according to the manufacturer's instruction using 0.35 kUA/L as the cutoff for a positive test.
Data on the national PHO consumptions summarized for the years 2008-2010 were taken from the United Nations International Narcotics Control Board database (www.incb.org).
Serum samples from 93 to 200 atopic individuals were tested for IgE-antibodies to PHO, MOR, and SUX. In Australia 10% were positive to PHO and 8.6% to MOR compared to 0.8% in Japan and 1.0 and 0.5%, respectively, in Korea (Table 1). Also the prevalence of IgE-sensitization to SUX was high in Australia, 4.3%, compared to 1.5% in Japan and 0.5% in Korea.
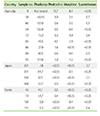
Of the 4 SUX-positive sera, in Australia all were positive to PHO but not the one in Korea. Interestingly, although the two SUX positive Japanese sera had notably high concentrations of IgE-antibodies, 2.1 and 3.7 kUA/L, respectively, they were negative to PHO (Table 2). Further, of the 8 MOR-positive sera in Australia, only 4 were SUX-positive.
IgE values were not available for the Korean samples and not for the Australian sample no. 85. Sample no. 95 was high but it is not known if it was higher than 10 000 kUA/L (Table 2). However, all sera had a Phadiatop value below 120 kUA/L.
PHO consumption in Australia was high (22.0 kg per million of inhabitants per year), whereas in Japan and Korea no PHO-containing cough syrups were available. In the Australian study population 53 of 93 (57%) indicated that they were frequent consumers of such cough mixtures.
This sero-epidemiological study of atopic subjects from Australia, Japan, and Korea shows that there is a positive relationship between national PHO consumption and IgE-sensitization to PHO. Thus, the present data supports the findings of a previous multinational study [1]. The comparison with Japan and Korea furthermore supports the PHO hypothesis; it is the exposure to PHO that induces an extraordinary production of IgE-antibodies to PHO, and its mother molecule MOR.
PHO and MOR carry two, noncross reacting IgE-binding epitopes. One is the QAI shared with the NMBAs, and the other an unknown structure, not shared with NMBAs [2]. IgE-antibodies to PHO and MOR are directed specifically to one of these two epitopes, inferring that not all IgE-antibodies to PHO or MOR, probably only about between 1/3-1/10 (unpublished observation), will also bind to SUX, i.e., to NMBAs [2, 3]. Therefore, using PHO or MOR to screen for IgE-antibodies to NMBAs, at least in populations like those in this study, will result in a considerable fraction of non-NMBA related positives. This is also confirmed by the present study where 5 of the 9 PHO or MOR positive Australian sera were SUX negative (Table 2). Disturbingly, however, an individual with IgE-antibodies to QAI seems to have a 200-300 times higher risk of experiencing anaphylaxis when exposed to SUX [3]. As a consequence reported anaphylactic reactions to NMBAs are more than 10 times as frequent in Australia (high consumption) as in Japan (no consumption).
In Australia, the problem with anaphylaxis to NMBAs and its link to IgE-sensitization to QAI common to available NMBAs was noticed many years ago [7, 8]. The present study supports this relationship. A serious challenge with such studies is, however, the spontaneous reporting of drug reactions that might vary considerably with time and between countries. Thus, published figures on prevalences of anaphylaxis must be evaluated carefully. However, despite this challenge it is becoming increasingly clear that anaphylaxis to NMBAs is more common in countries where PHO-containing cough syrups are available than in countries without these preparations.
Exposed to similar worrying findings, the Norwegian manufacturer decided not to prolong the marketing license and the cough syrup (Tuxi, Weifa AS, Oslo, Norway) was withdrawn in March, 2007. Within 3 years, the prevalence of IgE-sensitization and annual number of reported NMBA-related cases of anaphylaxis dropped significantly [3].
One problem with determination of IgE-antibodies to PHO, MOR, and SUX is that IgE-sensitized individuals taking a PHO-containing cough syrup will get a remarkable polyclonal booster of the serum IgE concentration [4, 5] which might cause false positive tests. Two of the Australian samples have high serum IgE levels but it is not known if they are below 10,000 kU/L, but both samples are negative, i.e., < 0.35 kUA/L, to SUX, which excludes nonspecific binding to PHO and MOR. In addition their Phadiatop values were not extremely high.
Another problem is the identification of relevant allergenic epitopes on NMBAs when looking for IgE-sensitization. The definition of an allergenic structure on, within or incorporating, a small chemical compound like a drug is complicated. The epitope can be the drug as such but also an in vivo breakdown product or a structure based on the small hapten molecule and its carrier. The latter was found in anaphylaxis to Patent Blue V where the only allergen detected triggering the patient's basophils was Patent Blue V and the patient's plasma [9].
Since there are so many chemicals in our environment carrying the QAI it is most likely that there must be sensitizers other than PHO. Thus, 4.6 folds higher prevalence of IgE-antibodies to QAI was recently reported among hairdressers than controls [10]. The finding that 2 sera from Japan had quite high concentrations of IgE-antibodies to the NMBA SUX, but no IgE-antibody to PHO or MOR, supports this hypothesis. However, the dramatic drop in the number of cases with anaphylaxis in Norway after withdrawal of the PHO-containing cough syrup, and the similar observation in Sweden [11], strongly supports a dominant role of PHO. In addition, the prevalence of anaphylaxis in the highly PHO-consuming Australia is more than 10 times higher than in PHO-free Japan. However, IgE-binding to an allergenic epitope other than QAI and a sensitizing agent other than PHO cannot be excluded.
The proposed relationship between exposing a population to PHO-containing cough syrups, IgE-sensitization and anaphylaxis to NMBAs was discussed by the EMA, in September, 2011. They did not find the results from Scandinavia a sufficient indication of the cause-effect-relationship [6]. Very few IgE-related adverse events have indeed been reported for PHO-containing drugs, which in our view is to be expected since PHO and MOR, as discussed above, are allergenically monovalent [2] and thus cannot trigger mast cells or basophils to initiate an allergic reaction. EMA stated that "the benefits of PHO-containing medicines continue to outweigh their risks" [6]. The remarkable effect on IgE production in individuals IgE-sensitized to PHO taking minute amounts of PHO cough syrup, so far seen only in graft versus host disease and cytostatic treatment of lymphadenopathy [12], is not mentioned. Neither were the well documented effects on IgE-sensitization and decline in anaphylaxis reports after withdrawal, from the Scandinavian markets, of PHO-containing drugs sufficiently recognized [3, 11]. The ethics of recommending "patients taking PHO-containing medicines can continue to do so" can be questioned especially since the antitussive effects of PHO in clinical studies at best seems to be marginal [13] and cough is rarely a serious disease. In Australia, the Therapeutic Goods Administration has recently taken the same position on the issue as the EMA despite the problem with NMBA-induced anaphylaxis reported in that country [14].
In summary, the present results support the PHO hypothesis. The data from Japan in addition points to our previous suggestion that other environmental chemicals than PHO or, probably rarely, repeated exposure to the NMBA [15] may give rise to IgE-antibodies to an NMBA [1]. However, at present these chemical factors remain unknown, seemingly operative to a much lesser degree than PHO and showing a clearly different pattern of sensitization.
Figures and Tables
Table 1
Summary of IgE-sensitization to pholcodine, morphine, and suxamethonium in sera from atopic individuals from Australia, Japan, and Korea
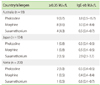
Table 2
Individual immunoglobulin E (IgE)-antibody results, in kUA/L, from the positive sera in Table 1, i.e., with IgE-antibody levels to pholcodine, morphine, and suxamethonium ≥0.35 kUA/L
ACKNOWLEDGEMENTS
We thank the following people for their contribution to the study: Ms Pamela Burton and Mrs Fiona Perram arranged collection of sera from allergy clinic patients in Australia.
References
1. Johansson SG, Florvaag E, Oman H, Poulsen LK, Mertes PM, Harper NJ, Garvey LH, Gerth van Wijk R, Metso T, Irgens A, Dybendal T, Halsey J, Seneviratne SL, Guttormsen AB. National pholcodine consumption and prevalence of IgE-sensitization: a multicentre study. Allergy. 2010; 65:498–502.

2. Florvaag E, Johansson SG, Oman H, Venemalm L, Degerbeck F, Dybendal T, Lundberg M. Prevalence of IgE antibodies to morphine. Relation to the high and low incidences of NMBA anaphylaxis in Norway and Sweden, respectively. Acta Anaesthesiol Scand. 2005; 49:437–444.

3. Florvaag E, Johansson SG, Irgens A, de Pater GH. IgE-sensitization to the cough suppressant pholcodine and the effects of its withdrawal from the Norwegian market. Allergy. 2011; 66:955–960.

4. Florvaag E, Johansson SG, Oman H, Harboe T, Nopp A. Pholcodine stimulates a dramatic increase of IgE in IgE-sensitized individuals. A pilot study. Allergy. 2006; 61:49–55.

5. Harboe T, Johansson SG, Florvaag E, Oman H. Pholcodine exposure raises serum IgE in patients with previous anaphylaxis to neuromuscular blocking agents. Allergy. 2007; 62:1445–1450.

6. European Medicines Agency. European Medicines Agency confirms positive benefit-risk balance of pholcodine-containing cough medicines [Internet]. London: European Medicines Agency;c1995-2014. cited 2013 Dec 12. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2011/11/news_detail_001389.jsp&mid=WC0b01ac058004d5c1.
7. Currie M, Webb RK, Williamson JA, Russell WJ, Mackay P. The Australian Incident Monitoring Study. Clinical anaphylaxis: an analysis of 2000 incident reports. Anaesth Intensive Care. 1993; 21:621–625.
8. Baldo BA, Fisher MM. Substituted ammonium ions as allergenic determinants in drug allergy. Nature. 1983; 306:262–264.

9. Johansson SG, Nopp A, Oman H, Stahl-Skov P, Hunting AS, Guttormsen AB. Anaphylaxis to Patent Blue V. II. A unique IgE-mediated reaction. Allergy. 2010; 65:124–129.

10. Dong S, Acouetey DS, Gueant-Rodriguez RM, Zmirou-Navier D, Remen T, Blanca M, Mertes PM, Gueant JL. Prevalence of IgE against neuromuscular blocking agents in hairdressers and bakers. Clin Exp Allergy. 2013; 43:1256–1262.

11. Johansson SG, Oman H, Nopp A, Florvaag E. Pholcodine caused anaphylaxis in Sweden 30 years ago. Allergy. 2009; 64:820–821.
12. Ringden O, Persson U, Johansson SG. Are increased IgE-levels a signal of an acute graft-versus-host reaction? Immunol Rev. 1983; 71:57–75.

13. Smith SM, Schroeder K, Fahey T. Over-the-counter medications for acute cough in children and adults in ambulatory settings. Cochrane Database Syst Rev. 2008; (1):CD001831.

14. Fisher MM, Baldo BA. The incidence and clinical features of anaphylactic reactions during anesthesia in Australia. Ann Fr Anesth Reanim. 1993; 12:97–104.
15. Johansson SG, Oman H, Degerbeck F, Tunelli J, Florvaag E, Nopp A. Anaphylaxis to atracurium: a non-QAI-dependent reaction? Acta Anaesthesiol Scand. 2012; 56:262–263.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download