Abstract
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare, but life-threatening, severe cutaneous adverse reactions most frequently caused by exposure to drugs. Several reports have associated the use of acetaminophen with the risk of SJS or TEN. A typical interval from the beginning of drug therapy to the onset of an adverse reaction is 1-3 weeks. A 43-year-old woman and a 60-year-old man developed skin lesions within 3 days after administration of acetaminophen for a 3-day period. Rapid identification of the symptoms of SJS and TEN caused by ingestion of acetaminophen enabled prompt withdrawal of the culprit drug. After administration of intravenous immunoglobulin G, both patients recovered fully and were discharged. These two cases of rapidly developed SJS/TEN after ingestion of acetaminophen highlight the possibility that these complications can develop within only a few days following ingestion of over-the-counter medications such as acetaminophen.
Acetaminophen is a widely used analgesic and anti-pyretic drug with no anti-inflammatory effects. Although hypersensitivity to acetominophen is rare, cutaneous reactions, such as urticaria, angioedema and maculopapular eruption, bronchospasm, and anaphylaxis have been described following exposure to the drug. Ingestion of acetaminophen has also been associated with systemic reactions, including vasculitis, hepatitis with glomerulonephritis, and possibly hypersensitivity syndrome, toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS) [1-4].
Both SJS and TEN are uncommon and life-threatening severe cutaneous adverse reactions most frequently caused by exposure to drugs [5]. One study reported that ingestion of acetaminophen was implicated in 48 of 245 patients with suspected SJS and TEN [6]. Both diseases are characterized by widespread epidermal necrosis, resulting in flaccid bullae with epidermal sloughing and frequent involvement of the mucous membrane. Although it is not clear whether SJS and TEN are indeed separate diseases or simply variants of a related disorder, both generally develop within at least several days after the initial administration of the causative drug [7]. However, here we describe two cases of SJS/TEN where symptoms were apparent within only two or three days after ingestion of acetaminophen.
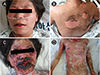
A 43-year-old woman visited our hospital with an erythematous skin rash involving bullae throughout the body. Oropharyngeal ulceration and conjunctivitis developed within just two days after medication with acetaminophen to treat an infection of the upper respiratory tract (Fig. 1). The patient had a history of asthma, which had been controlled for five years by inhalation of a corticosteroid or long-acting beta-2 agonist, and had reported an incident of immediate drug hypersensitivity, urticaria to acetaminophen one year earlier. On examination, the patient was alert and appeared acutely ill. Her body temperature was 39.4℃, blood pressure was 115/90 mmHg, pulse rate was 140 beats per minute, and respiratory rate was 24 breaths per minute. Macular rash and epidermal detachment was evident on approximately 20% of the body surface. Nikolsky's sign was positive. Tenderness was evident, especially in parts of the skin affected by the rash. The white blood cell count was 2.900/mm3, the hemoglobin concentration was 13.2 g/dL, and the platelet count was 160,000/mm3. Her C-reactive protein was increased to 16.64 mg/dL, and the level of procalcitonin was 0.34 ng/mL. The level of total protein was 5.9 g/dL, the level of albumin was 3.1 g/dL, the level of blood urea nitrogen was 8 mg/dL, and the level of serum creatinine was 0.6 mg/dL. The level of aspartate aminotransferase was 102 IU/L, and the level of alanine aminotransferase was 40 IU/L. Levels of sodium, potassium and calcium levels were slight decreased. There was no eosinophilia.
The skin lesions rapidly progressed to bullous skin lesions covering the whole body, with epidermal detachment apparent over more than 30% of the body surface with time in spite of administration of high dose systemic corticosteroid (Fig. 1). After diagnosis of TEN, we administered intravenous immunoglobulin G (IVIG) at 2 g/kg/day for 3 days. After the treatment with IVIG, the epidermal detachment of the skin ceased and the severity of the skin lesions decreased. Empirical antibiotic use was continued for three weeks. The patient was discharged from the hospital 33 days after admission.
We thoroughly investigated other causes of the pathology, including viral infection. Nonetheless, there was no evidence of other causes besides ingestion of acetominophen.
A 60-year-old man with a five-year history of diabetes mellitus and hypertension was treated with acetaminophen for three days owing to infection of the upper respiratory tract. He had no history of allergic responses. A erythematous rash throughout the body was evident 3 days after initial exposure to acetominophen. The rash spread and became generalized within a few days, with the appearance of bullae. He was admitted to another hospital 7 days after initial exposure to acetaminophen (day 7) and treated with IVIG delivered at a rate of 1 g/kg/day for 3 days. Skin lesions were accompanied by a fever, with a body temperature > 39℃. A skin biopsy performed on day 8 indicated an early phase of toxic epidermal necrolysis. On day 14 he was referred to our hospital with multiple exfoliations, extensive bullae throughout his body, oral mucositis and ocular involvement (Fig. 2). On examination, the patient was alert and appeared chronically ill. His body temperature was 38.4℃, blood pressure was 144/70 mmHg, pulse rate was 90 beats per minute, and respiratory rate was 20 breaths per minute. Nikolsky's sign was positive. Tenderness was evident, especially in parts of the skin affected by the rash.
The patient's white blood cell count was 1.700/mm3, the hemoglobin concentration was 11.9 g/dL, and the platelet count was 183,000/mm3. His C-reactive protein was increased to 6.55 mg/dL and the level of procalcitonin was 0.1 ng/mL. The level of total protein was 4.7 g/dL, the level of albumin was 1.5 g/dL, the level of blood urea nitrogen was 10 mg/dL, and the level of serum creatinine was 0.6 mg/dL. The sodium, potassium, phosphate, calcium and uric acid levels were slightly decreased. There was no evidence of eosinophilia and levels of liver enzymes were within the normal range.
The patient was isolated to reduce the risk of secondary infection, and received fluid therapy and parenteral nutrition with high protein content. After treatment of skin lesions by the topical application of mupirocin, 0.9% NaCl and 0.5% AgNO3 three times a day for 7 days, the skin condition gradually improved. The patient was discharged from the hospital on day 30. We are confident that the reaction was caused by acetaminophen, because it appears to be the only drug to which the patient was exposed to within a 30-day period prior to his initial admission.
Hypersensitivity to acetaminophen is rare, although several instances involving skin manifestations are reported, including SJS and TEN. For instance, the onset of erythema multiforme-SJS after paracetamol ingestion was confirmed by a rechallenge test involving this drug [8]. In another case, which involved a seven-year-old girl who developed TEN after acetaminophen ingestion, oral rechallenge with acetaminophen caused a similar eruption [9].
The median latency time between the beginning of use and onset of either SJS or TEN is less than 4 weeks for most drugs. The reported times are 15 days for carbamazepine, 24 days for phenytoin, 17 days for phenobarbital, and 20 days for allopurinol. In general, an interval of 4-28 days between the beginning of drug use and the onset of the adverse reaction is most suggestive of an association between the medication and the onset of either SJS or TEN [10]. However, our two patients developed SJS and TEN within only 3 days after ingestion of acetaminophen, which is far more rapidly than for most reported cases. This appears to be the first report to reveal that both SJS and TEN can be developed within a very short period after administration of acetominophen. Therefore, physicians should always consider the potential roles of drugs taken even within a few days before the onset of symptoms onset when attempting to identify the agent responsible for either SJS or TEN. The fact that our first patient had a previous history of mild hypersensitivity to acetaminophen suggests that a more rapid response may occur upon re-challenge with a drug [5, 7]. However, in the second case, there was no previous history of drug hypersensitivity. Probably, the mechanism of earlier onset may be associated with sudden changes in mediators and expression of arachidonic acid after acetaminophen was administered. In fact, there have been no researches on the earlier onset of SJS or TEN.
Owing to the severity of the cutaneous adverse drug reaction that can be associated with TEN, which can be fatal, its diagnosis is considered to constitute a medical emergency. The average reported mortality is 25-35%, although this can be even higher in elderly patients and those for whom epidermal detachment covers a large surface area. The Score of TEN (SCORTEN) disease scoring system can be used to predict mortality [11]. For instance, because the first patient was 43-year-old, epidermal detachment was observed on 30% of the skin surface area, and the heart rate was 140 beats per minute, the SCORTEN score was 3, and the predicted mortality was 35.3%. The second patient was 60 years old and epidermal detachment was evident on at least 80% of the skin surface area. The heart rate was lower than 100 beats per minute. His SCORTEN score was 2, and the predicted mortality was 12.1%.
As soon as diagnosis of TEN has been established, prompt identification and withdrawal of the culprit drug is essential. Early withdrawal of the causative drug is associated with a better prognosis for patients with either TEN or SJS [12]. After withdrawal of the causative drug, supportive care is an essential part of the therapeutic approach, including management of fluid and electrolytes. If the patient has insufficient oral intake, parenteral nutrition with a high protein content or oral nutrition by nasogastric tube is required. Measures to ensure an aseptic and temperature-controlled environment are conducive to effective treatment of TEN. Systemic corticosteroid use was the standard treatment until the early 1990's, although no benefit has been found in controlled trials. Corticosteroids did not show a significant effect on mortality in comparison with supportive care only [13]. Our first patient was treated with systemic corticosteroids because she presented symptoms typical of both SJS and TEN, although the therapy failed.
Recent evidence that the Fas-Fas ligand pathway of apoptosis is the first, or at least a pivotal, step in the pathogenesis of TEN, prompted investigation of the feasibility of using IVIG for the treatment of TEN. In vitro demonstration that IVIG can inhibit cell death and the interaction of Fas (CD95) and FasL (CD95L) provides a rationale for its use in humans, and non-controlled studies suggest possible benefits associated with the use of IVIG [14, 15]. Comparison of published data suggests that total IVIG doses of more than 2 g/kg may be of greater benefit than doses of 2 g/kg or less [16]. Our first patient received a 2 g/kg/day dose of IVIG in our hospital, whereas the second patient received a 1 g/kg/day dose for 3 days in another hospital. Whereas epidermal detachment of the skin ceased and the frequency of skin lesions decreased after the first patient received IVIG therapy, infusion of IVIG did not arrest the progress of disease in the second patient. Probably, other factors unrelated to the inflammation soothed by IVIG administration may affect the course of disease.
Besides the skin, TEN is also frequently associated with symptoms that affect the eyes and mucous membranes of the oral, gastrointestinal, pulmonary, genital, and urinary tracts. Therefore, an interdisciplinary strategy is essential for follow-up procedures and the treatment of sequelae. Early intervention by an ophthalmologist is essential to assess the extent to which the eyes are affected, and may necessitate prompt treatment by topical application of steroids. Long-term complications, such as hypopharyngeal stenosis combined with dysphagia and esophageal strictures, are difficult to treat and may require laryngectomy. More than 50% of patients surviving TEN suffer from long-term sequelae of the disease.
Given that rechallenge tests with medication are contra-indicated for SJS and TEN, we did not seek to test the effects of rechallenge in either of our patients.
In summary, this report of two cases where SJS and TEN developed rapidly after ingestion of acetaminophen underscores the importance that physicians bear in mind that SJS and TEN can be developed within only a few days after ingestion of over-the-counter medications, such as acetaminophen.
Figures and Tables
ACKNOWLEDGEMENTS
This study was supported by a grant of the National Project for Personalized Genomic Medicine, Ministry for Health & Welfare, Republic of Korea (A111218-PG01).
References
1. Kvedariene V, Bencherioua AM, Messaad D, Godard P, Bousquet J, Demoly P. The accuracy of the diagnosis of suspected paracetamol (acetaminophen) hypersensitivity: results of a single-blinded trial. Clin Exp Allergy. 2002; 32:1366–1369.

2. Vidal C, Pérez-Carral C, González-Quintela A. Paracetamol (acetaminophen) hypersensitivity. Ann Allergy Asthma Immunol. 1997; 79:320–321.
3. Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah H, Tripathi C. Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: a multicentric retrospective study. J Postgrad Med. 2011; 57:115–119.

4. Levi N, Bastuji-Garin S, Mockenhaupt M, Roujeau JC, Flahault A, Kelly JP, Martin E, Kaufman DW, Maison P. Medications as risk factors of Stevens-Johnson syndrome and toxic epidermal necrolysis in children: a pooled analysis. Pediatrics. 2009; 123:e297–e304.

5. Lee T, Bae YJ, Park SK, Park HJ, Kim SH, Cho YS, Moon HB, Lee SO, Kim TB. Severe pneumonia caused by combined infection with Pneumocystis jiroveci, parainfluenza virus type 3, cytomegalovirus, and Aspergillus fumigatus in a patient with Stevens-Johnson syndrome/toxic epidermal necrolysis. Acta Derm Venereol. 2010; 90:625–629.
6. Roujeau JC, Kelly JP, Naldi L, Rzany B, Stern RS, Anderson T, Auquier A, Bastuji-Garin S, Correia O, Locati F, Mockenhaupt M, Paoletti C, Shapiro S, Shear N, Schöpf E, Kaufman DW. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995; 333:1600–1607.

7. Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med. 1994; 331:1272–1285.

8. Trujillo C, Gago C, Ramos S. Stevens-Jonhson syndrome after acetaminophen ingestion, confirmed by challenge test in an eleven-year-old patient. Allergol Immunopathol (Madr). 2010; 38:99–100.

9. Halevi A, Ben-Amitai D, Garty BZ. Toxic epidermal necrolysis associated with acetaminophen ingestion. Ann Pharmacother. 2000; 34:32–34.

10. Mockenhaupt M. The current understanding of Stevens-Johnson syndrome and toxic epidermal necrolysis. Expert Rev Clin Immunol. 2011; 7:803–813.

11. Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010; 5:39.

12. Garcia-Doval I, LeCleach L, Bocquet H, Otero XL, Roujeau JC. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000; 136:323–327.

13. Schneck J, Fagot JP, Sekula P, Sassolas B, Roujeau JC, Mockenhaupt M. Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: A retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol. 2008; 58:33–40.

14. Mangla K, Rastogi S, Goyal P, Solanki RB, Rawal RC. Efficacy of low dose intravenous immunoglobulins in children with toxic epidermal necrolysis: an open uncontrolled study. Indian J Dermatol Venereol Leprol. 2005; 71:398–400.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download