Abstract
Lamotrigine is a recent medication which is prescribed for various neuropsychiatric conditions. It is generally well-tolerated, but recent pharmacoepidemiological evidence suggests that lamotrigine is associated with risks of developing severe cutaneous drug reactions like toxic epidermal necrolysis (TEN). However, there still remains the diagnostic challenge regarding how to confirm the drug causality in suspected cases. In most cases so far, lamotrigine causality has not been objectively demonstrated, which was possibly due to high risk of oral challenge tests or the lack of useful in vitro drug assays. Here we report a case of lamotrigine-induced TEN, of which the drug causality was confirmed by in vitro granulysin and cytokine assays.
Toxic epidermal necrolysis (TEN) is a rare fatal disease with unclear pathogenic mechanisms [1, 2]. Lamotrigine is one of recent medications for neuropsychiatric disorders with well tolerability [3], but accumulating evidence suggests that lamotrigine has potential risks of severe cutaneous reactions like TEN [4]. However, to date, the drug causality in TEN cases has been mostly determined in clinical context or relied on scoring system like ALDEN algorithm [5], which was due to high risk of rechallenge tests [6] or low sensitivity of in vitro lymphocyte transformation test (LTT) in TEN [7]. Here we report a case of lamotrigine-induced TEN, of which the drug causality was objectively confirmed by in vitro granulysin and cytokine assays.
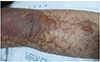
A 22-year-old female with borderline personality disorder presented with generalized bullous eruption that started one week ago. She was prescribed lamotrigine for 40 days prior to the onset, but also took massive lamotrigine overdose 4 days ago. At admission, her temperature was 37.3℃ with blood pressure 129/69 mmHg, heart rate 90 beats/min and respiration rate 20 breaths/min. On examination, bullous formation and skin detachment was found in more than 50% of body surface (Fig. 1). Mucosal involvement was found in conjunctiva, oral cavity, and vagina. Nikolsky's sign was also positive. Initial laboratory investigation showed albumin 3.4 g/dL, blood urea nitrogen 12 mg/dL, glucose 141 mg/dL, bicarbonate 27.2 mmol/L, and C-reactive protein 2.53 mg/dL. Viral serologic examination found no abnormality for hepatitis virus, or human immunodeficiency virus. Serum human herpesvirus-6, cytomegalovirus, or Epstein-Barr virus titer was also normal by real-time polymerase chain reaction.
Based on clinical findings, she was diagnosed as TEN. Medication history revealed that lamotrigine was the only agent which had been added during the past few months, and thus was suggested to be the culprit. Initial treatment included the discontinuation of lamotrigine, intravenous methylprednisolone 1 mg/kg/day, and parenteral nutritional and fluid therapy. In addition, intravenous immunoglobulin (IVIG) 1 g/kg/day was administered for 5 days. Her skin lesions were managed with wet dressing, and ocular and genital lesions were treated by ophthalmologists and gynecologists respectively.
During the first week since admission, overall signs started to improve; no further fever or active bullous formation were observed. The IVIG therapy was stopped on hospital day 5 as scheduled. However, on day 6, she abruptly showed signs of aggravation; she redeveloped fever, oral mucosal bleeding, and new extensive bullous eruptions at trunk and extremities. As her deterioration was rapid despite ongoing systemic corticosteroid therapy, we decided to add intravenous cyclosporine 3 mg/kg/day. On day 8, her fever and eruptions began to resolve. Since then, she recovered without further aggravation, and was discharged on hospital day 33. Methylprednisolone was slowly tapered until day 21. Cyclosporine was maintained for 4 weeks, and tapered off.
To prevent re-exposure, we proceeded in vitro investigations to confirm the drug causality. After one month since off treatment, we obtained her peripheral blood mononuclear cells (PBMCs) to stimulate with lamotrigine. As granulysin is a key mediator in TEN [8], we did experiments to measure in vitro granulysin responses upon lamotrigine stimulation. Briefly, her PBMCs were cultured with or without 20 or 50 µg/mL lamotrigine for 4 days. To determine granulysin expression in different subsets, PBMCs were fixed, permeabilized, and stained with anti-CD3, anti-CD4, anti-CD8, anti-CD56, and antigranulysin antibodies; the results were measured by flow cytometry. As results, we found up-regulation of granulysin in CD3-CD56+NK (natural killer) cells in the patient, at day 4 upon lamotrigine stimulation (Fig. 2). Granulysin up-regulation was not found in CD4+ or CD8+T cells at day 4. In addition, we observed high increase of interferon-γ (IFN-γ) in the supernatants (Table 1). All the experiments included three healthy volunteers as negative controls, and were duplicated. Collectively, our experiments confirmed lamotrigine as the offending agent.
Since the first case report in 1990s [9], lamotrigine has been considered as one of significant causes for Stevens-Johnson syndrome (SJS)/TEN among recently marketed medication [4]. Despite the epidemiological associations, there still remains the diagnostic challenge regarding how to confirm the causality in lamotrigine-suspected TEN cases [7].
Here we objectively demonstrated the lamotrigine causality by measuring in vitro granulysin expression in CD56+NK cells. Granulysin is a key mediator, and NK cell is a main source [8, 10, 11]. Our positive findings are in line with recent literature, suggesting potential utility of in vitro drug assays directed to endotypes [7, 12]. The LTT was insensitive in lamotrigine-SJS/TEN cases (4/23), even less sensitive than in mild eruption (3/6) [7]. As SJS/TEN involves specific cytotoxic process rather than general lympho-proliferative features, the low sensitivity of LTT may be plausible and the specific assay could be more appropriate.
However, one single in vitro test may not be always sufficient. Accordingly, Porebski et al. [12] recently suggested to combine different assays to identify causative drugs in 15 SJS/TEN patients. We generally agree with their opinions as with our findings of concomitant IFN-γ increase in the supernatants. However, we partly argue against their granulysin measurement in CD4+T cells. The primary reason is that the degree of granulysin up-regulation in CD4+T cells was too small (0.65%) to detect significant difference from the baseline value, which was in contrast to 14.1% up-regulation in NK cells [12].
In clinical aspects, we briefly discuss the therapeutic utility of cyclosporine, considering the lack of proven therapy in TEN [13]. So far, several immune-modulating modalities have been tried, such as IVIG [14], corticosteroids [15], or plasmapheresis [16]. As TEN had similar histological findings with graft versus host disease [17], cyclosporine therapy has been suggested [18]. Recent open trials evaluated the efficacy of cyclosporine 1.5 mg/kg/day therapy in 29 SJS/TEN patients, and found trends toward reducing mortality (0 in the cyclosporine-treated group vs. 2.75 by SCORTEN prediction) and promote skin stabilization [19]. We consider cyclosporine as a potential therapeutic option in progressive TEN cases, and controlled trials are necessary.
Collectively, we report a case of lamotrigine-induced TEN, in which the causality was confirmed by in vitro granulysin and cytokine assays. We expect in vitro granulysin and cytokine assays to be further utilized in identifying the culprit drug in patients who develop SJS/TEN while taking multiple drugs, or in studying cross-reactivity among related-drugs.
Figures and Tables
 | Fig. 2Flow cytometric analyses of granulysin expression in CD3-CD56+ natural killer cells from a toxic epidermal necrolysis patient and a healthy control. Intracellular expression of granulysin was detected in CD3-CD56+ natural killer cells, at day 4 upon lamotrigine in vitro stimulation. The numbers represent the percentages of the dots in each gated area. |
ACKNOWLEDGEMENTS
The present study was supported by a grant No. 04-2012-0980 (2012-1336) from the SNUH Research Fund.
References
1. Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part I. Introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013; 69:173.e1–173.e13.
2. Song WJ, Chang YS. Drug hypersensitivity syndrome: a literature review of associated viral reactivation. Korean J Asthma Allergy Clin Immunol. 2011; 31:77–83.
3. Messenheimer J, Mullens EL, Giorgi L, Young F. Safety review of adult clinical trial experience with lamotrigine. Drug Saf. 1998; 18:281–296.

4. Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, Sidoroff A, Schneck J, Roujeau JC, Flahault A. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol. 2008; 128:35–44.

5. Sassolas B, Haddad C, Mockenhaupt M, Dunant A, Liss Y, Bork K, Haustein UF, Vieluf D, Roujeau JC, Le Louet H. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther. 2010; 88:60–68.

6. Gomez E, Torres MJ, Mayorga C, Blanca M. Immunologic evaluation of drug allergy. Allergy Asthma Immunol Res. 2012; 4:251–263.

7. Tang YH, Mockenhaupt M, Henry A, Bounoua M, Naldi L, Le Gouvello S, Bensussan A, Roujeau JC. Poor relevance of a lymphocyte proliferation assay in lamotrigine-induced Stevens-Johnson syndrome or toxic epidermal necrolysis. Clin Exp Allergy. 2012; 42:248–254.

8. Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, Chin SW, Chiou CC, Chu SC, Ho HC, Yang CH, Lu CF, Wu JY, Liao YD, Chen YT. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008; 14:1343–1350.

9. Wadelius M, Karlsson T, Wadelius C, Rane A. Lamotrigine and toxic epidermal necrolysis. Lancet. 1996; 348:1041.

10. Tewary P, Yang D, de la Rosa G, Li Y, Finn MW, Krensky AM, Clayberger C, Oppenheim JJ. Granulysin activates antigen-presenting cells through TLR4 and acts as an immune alarmin. Blood. 2010; 116:3465–3474.

11. Hashizume H. Recent progress of elucidating the mechanisms of drug hypersensitivity. Asia Pac Allergy. 2012; 2:203–209.

12. Porebski G, Pecaric-Petkovic T, Groux-Keller M, Bosak M, Kawabata TT, Pichler WJ. In vitro drug causality assessment in Stevens-Johnson syndrome - alternatives for lymphocyte transformation test. Clin Exp Allergy. 2013; 43:1027–1037.
13. Thong BY. Stevens-Johnson syndrome/toxic epidermal necrolysis: an Asia-Pacific perspective. Asia Pac Allergy. 2013; 3:215–223.
14. Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, Hunziker T, Saurat JH, Tschopp J, French LE. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 1998; 282:490–493.

15. Kardaun SH, Jonkman MF. Dexamethasone pulse therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis. Acta Derm Venereol. 2007; 87:144–148.

16. Chaidemenos GC, Chrysomallis F, Sombolos K, Mourellou O, Ioannides D, Papakonstantinou M. Plasmapheresis in toxic epidermal necrolysis. Int J Dermatol. 1997; 36:218–221.

17. Peck GL, Herzig GP, Elias PM. Toxic epidermal necrolysis in a patient with graft-vs-host reaction. Arch Dermatol. 1972; 105:561–569.

18. Arevalo JM, Lorente JA, Gonzalez-Herrada C, Jimenez-Reyes J. Treatment of toxic epidermal necrolysis with cyclosporin A. J Trauma. 2000; 48:473–478.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download