Abstract
Human airways contact with pathogen-associated molecular patterns and danger-associated molecular patterns present in many environments. Asthmatic's airways may be more susceptible to these patterns and lead to inflammasome activation; however, the participation of inflammasome in the development and exacerbation of asthma is not fully understood and remains controversial. Asthma is a heterogeneous group composed of different airway inflammation patterns with different underlying immune mechanisms. One mechanism is neutrophilic airway inflammation based on the axis of inflammasome activation, interleukin (IL) 1β/IL-18 production, T helper 17 activation, IL-8/IL-6 overproduction, and neutrophilic inflammation. The role of inflammasome activation has been highlighted in experimental asthma models and some evidence of inflammasome activation has been recently demonstrated in human neutrophilic asthmatic airways. In addition to caspase-1 activation, proteinase 3 and other protease from activated neutrophils directly cleave pro-IL-1β and pro-IL-18 to IL-1β and IL-18, which contribute to the phenotype of subsequent adaptive immune responses without inflammasome activation. Data suggests that neutrophilics in asthmatic airways may have an additional effect in initiating inflammasome activation and amplifying immune responses. Among the mediators from neutrophils, S100A9 seems to be one candidate mediator to explain the action of neutrophils in amplifying the airway inflammation in concert with inflammasome.
Aeroallergens had been suggested as trigger factors for the development and exacerbation of asthma in 1860 [1]; consequently, the main immunologic mechanism of asthma was established as an axis of allergens, T helper 2 (Th2) lymphocytes, IgE, mast cells and eosinophils in the early 1990s [2]. Over half of adult asthmatics show negative skin prick reactivity to aeroallergens; therefore, the innate immune response emerged as a newly identified contributor to the immunopathogenesis of allergy and asthma in the mid-1990s [3]. Many inflammatory mediators are shown to be responsible for the maintenance and chronicity of inflammatory responses through the secretion of cytokines from epithelial, endothelial and constitutive mesenchymal cells in addition to immune cells [4]. Innate immune responses have also attracted significant attention as a candidate pathogenesis of asthma. Pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) are essential components of the innate immunity via activating several kinds of immune cells such as dendritic cells and activating inflammasome. However, the participation of inflammasome in the development and exacerbation of asthma has not been fully studied and remains controversial. We discuss the possible role of inflammasome in the development and exacerbation of asthma.
Pattern recognition receptors (PRRs) are expressed by innate immune cells that identify endogenous and exogenous PAMPs or DAMPs [4]. PRRs are divided into membrane-bound and cytoplasmic PRRs (Table 1). Membrane bound forms include Toll-like receptors (TLRs) and C-type lectin receptors. Nucleotide-binding oligomerization domains (NOD)-like receptors (NLRs) as well as RIG-I-like receptors (RLRs) located in the cytoplasm. Recognition of extracellular or endosomal PAMP is mediated by TLRs [5]. Lipopolysaccharide, cell components of gram negative bacteria, is one prototype of PAMP, and recognized by TLR4. Other PAMPs include bacterial flagellin (recognized by TLR5), lipoteichoic acid of gram positive bacteria, and single or double-stranded RNA and DNA of viruses (recognized by TLR3). Activation of TLRs induces nuclear factor (NF)-κB signaling to phosphorylate MAP kinase and the secretion of proinflammatory cytokines such as tumor necrosis factor (TNF)-α, IL-6, IL-8, pro-IL-1β, pro-IL-18 and costimulatory molecules (Fig. 1). Noninfectious DAMPs are released outside dyeing cells following tissue injury and amplify immune responses [6]. Protein DAMPs include heat-shock proteins, high-mobility group box1, and hyaluronan fragments. Nonprotein DAMPs include adenosine triphosphate (ATP), uric acid, heparin sulfate and DNA.
Approximately 20 cytoplasmic NLRs and RLR respond to DAMPs and PAMPs. NOD1 and NOD2 recognize constituents of gram-negative or positive bacteria. There are 14 members of NACHT, LRR and PYD domains-containing protein (NALP). The activation process of NOD-like receptor family, pyrin domain containing 3 (NLRP3) is well known and its ligands are infectious molecules that include muramyl dipeptide, toxins, bacterial DNA, and double stranded RNA as well as noninfectious materials such as silica, alum, extracellular ATP and uric acid crystals. PAMPs are initially recognized by TLRs that activate NF-κB resulting in transcription of pro-IL-1β and pro-IL-18. Subsequently, the NLRP3 inflammasome complex is assembled to activate caspase-1, which cleaves pro-IL-1β and pro-IL-18 into their mature IL-1β and IL-18 (Fig. 1). In addition, caspase-1 activation induces interferon (IFN)-γ secretion [7] and cleavage of IL-33 [8].
The administration of IL-1β induces airway hyperreactivity: a characteristic feature of asthma [9]. IL-1β is increased in the serum and bronchoalveolar lavage fluids of human asthmatics and decreases after steroid inhalation [10,11,12]. IL-18 levels are increased in theperipheral blood of asthmatics; however, controversial results have been reported [13,14,15]. The data suggest a possible presence of inflammasome activation in the asthmatic airways. Simpson et al. [16] recently proved the expression of NLRP3 and caspase-1 protein with a concomitant increase of IL-1β in macrophages as well as neutrophils in sputum of neutrophilic asthma.
Early exposure to microbes skews the immune response away from Th2 cytokines to prevent the development of IgE-dependent asthma [17]. The hygiene hypothesis raises the possible protective role of inflammasome against the development of asthma; however, current evidences favor a proinflammatory role of inflammasome activation. Components of virus, bacteria, and fungus are well known PAMPs. Double strand viral DNA activates the AIM2 inflammasome, while viral RNA such as Influenza A virus (IAV) triggers the assembly of the NLRP3 inflammasome [18]. Bronchial epithelial cells in asthmatics show an influenza-induced enhancement of caspase-1 activity [19]. In experimental mice, elderly ones have impairment of NLRP3 activity on IAV infection [20]. This data may extend to the high mortality of elderly subjects when they are infected with IAV. Similar to IAV, rhinovirus and respiratory syncytial virus trigger the NLRP4 inflammasome [21]. Chlamydia pneumonia, suspected as a causative agent for asthma chronicity, also activates the NLRP3/ASC (apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain) inflammasome [22].
Asthmatics have a different pattern of microbiome in the airways [23]. Interestingly, the lung microbial burden increases in asthma and chronic obstructive pulmonary disease and the extent of the total microbiomeis correlated to airway obstruction [24]. These data suggest that microbiome-induced inflammasome activation may contribute to asthma severity rather than the protective role of the hygiene hypothesis.
The critical role of inflammasome is highlighted in experimental asthma models rather than human asthmatics. During ovalbumin sensitization, alum potentiates the inflammatory response by activating the NLRP3 inflammasome [25]. The cytotoxic effect of alum adjuvant induces cell death and leakage of intracellular ATP or uric acid crystals and causes inflammasome activation [26]. Patients with asthma and chronic obstructive pulmonary disease have elevation of ATP as well as uric acid in their airways [27]; consequently, it is suggested that cell death occurs in the airways of symptomatic asthmatics or during prolonged exacerbation that may induce inflammasome activation.
Urban particulates and inorganic particles such as titanium dioxide, silica, and asbestos activate inflammasome [28]. PM10 or PM2.5 concentrations have been increasing, especially in developing countries and can contribute to increasing incidences and severity of asthma. However, the findings observed in animal models are yet to be validated in human asthmatic studies. Allen et al. [29] recently demonstrated that NLRP3 was not necessary in the development of allergic asthma models; however, they observed the effect of NLRP3 in Th2 type murine model of asthma. Subsequently, the Th1 or Th17 dominant asthma model is to be analyzed for the participation of inflammasome activation in different animal models of asthma.
IL-1β is synthesized by blood monocytes, tissue macrophages, and dendritic cell (DC) after activation by proinflammatory stimuli such as TNF or TLR ligands [30]. Inflammasomes are present in the myelomonocyte cell lineage; however, recent evidence indicates that several types of epithelial cells may also contain these structures [31]. IL-1β signaling regulates early Th17 cell differentiation via expressing interferon regulatory factor 4 and retinoic acid receptor-related orphan receptor gamma [32]; which is essential transcription factor in Th17 differentiation. IL-1β induces the development of IL-17-producing T cells in cooperation with IL-23 [33]. In contrast to IL-1β, IL-18 signaling affects IFN-γ producing Th1 cell differentiation in conjunction with IL-12 [34] and with IL-13 [35]. Among the multiple lymphocytes subsets, IL-1β and IL-18 in combination with the other cytokines may exert biologic effects on enhancement of Th17 cell differentiation in the lung leading to neutrophilic inflammation [36].
There are few studies on the relation of inflammasome activation with asthma phenotypes. Asthma is a heterogeneous group composed of different immunopathogenesis and clinical manifestations. Accordingly, a certain type of asthma may produce inflammasome activation as an underlying mechanism. Adult asthma can be classified into several subtypes: (1) early onset allergic asthma with specific IgE and Th2 cytokine dominance, (2) eosinophilic type of adult onset showing corticosteroid refractory, less allergic tendency but high IL-5 concentration, (3) exercise-induced mild intermittent type of Th2 dominancy and mast cell activation, (4) obesity - related adult onset without evidence of Th2 activation, and (5) neutrophilic types with low forced expiratory volume in 1 second and air trapping via Th17 pathway activation [37]. Adult onset asthma due to obesity may be related to the activation of inflammasome because inflammasome activation is observed in several metabolic disorders such as obesity [38]. Neutrophilic asthma is another group suspected of inflammasome activation. Airway neutrophilia is observed in noninfectious asthmatics, especially severe status asthmaticus [39]. Our previous studies indicate that refractory asthma is less allergic and had more intense airway neutrophilia compared to mild persistent asthma [40,41]. In the subgroup analysis of refractory asthma, airway neutrophilia is associated with severe air flow limitation [40]. The neutrophilic type (>70% of neutrophils in sputum) is more frequently observed in chronic progressive airway obstruction while eosinophilic type was predominantly observed in brittle types of asthma with frequent exacerbations rather than chronic progressive airway obstruction [41]. Moore et al. [42] recently reported that the multivariate approach identified 4 asthma sub-phenotypes that represent the severity and airway inflammation pattern. Among them, sputum neutrophil counts are associated with more severe asthma phenotypes. The extent of neutrophilic inflammation in asthmatic airways is closely correlated with gene and protein levels of IL-17A [43]. These data suggest that neutrophilic airway inflammation may be based on the axis of inflammasome activation-IL-1β/IL-18-IL-17-neutrophilic infiltration.
In addition, peripheral blood neutrophils express IL-17 protein and the levels are elevated in asthmatics [44]. In the analysis of sputum cells in neutrophilic asthma, NLRP3 and caspase-1 protein are expressed in neutrophils as well as macrophages [16]. Proteinase 3 from activated neutrophils during early responses directly cleaves pro-IL-1β and pro-IL-18 to IL-1β and IL-18 that contributes to the phenotype of subsequent adaptive immune responses independently from inflammasome activation [45]. These data suggest that neutrophils may exert an additional effect of initiating inflammasome activation and amplifying immune responses in asthmatic airways.
In allergic asthmatics, airway inflammation is triggered by the inhalation of specific allergens such as house dust mite allergens and pollens. DCs are essential for priming naive T cells towards Th2 differentiation; however, respiratory allergens also induce innate immune response via tight collaboration with PAMPs and DAMPs. Components of allergens trigger DCs in TLR4 or CTR dependent manner [46,47]. Without inflammasome activation, the triggering of these receptors on epithelial cells subsequently leads to the release of innate pro-Th2 cell cytokines that include thymic stromal lymphopoietin (TSLP), granulocyte-macrophage colony stimulating factor, IL-25, and IL-33 [48,49]. IL-33 represents a key cytokines in an innate immune response because it activates Th2 lymphocytes, eosinophils, basophils, and mast cells via up-regulation TSLP and IL-25 synthesis. Biologically active IL-33 is constitutively stored in the nuclei of human airway epithelial cells and endothelial cells and it can be released into the extracellular space after cellular damage [50,51]. Fungal allergen exposure induces an acute extracellular accumulation of ATP; autocrine ATP sustains increases in intracellular calcium concentration and releases IL-33 through the activation of P2 purinergic receptors [50]. Consequently, ATP and purinergic signaling in the respiratory epithelium are critical sensors for airway exposure to airborne allergens. IL-33 exerts its biological effect through similar signaling pathways that involve MyD88 and TRAF6 dependent pathways such as 1L-1β and IL-18, which are major end products of inflammasome activation. IL-33 stimulates Th2 cells through ST2 [52], a member of the IL-1 receptor family, which is expressed to various immune cells that include mast cells, basophils, eosinophils, Th2 lymphocytes, invariant natural killer T, natural killer cells, macrophages, dendritic cells, and neutrophils. IL-33 induces high amounts of Th2 cytokines by type 2 innate lymphoid cells (natural helper cells, nuocytes, or innate helper 2 cells) [53]. It was initially believed that processing of IL-33 by caspase-1 to a mature form was required for biological activity [54]. However, full-length IL-33 is biologically active and the processing of IL-33 by caspases results in its inactivation rather than activation [8,55].
Proteinase 3 from activated neutrophils is also noted to have dual functions on inactive human and mouse precursor IL-33 proteins [56]. Proteinase 3 activates the precursor form to biological active forms; however, the increase of incubation time with proteinase 3 abrogates IL-33 activities. The proteinase 3 directly cleaves pro-IL-1β and pro-IL-18 to IL-1β and IL-18 without inflammasome activation [45]. Considering together that neutrophils synthesize IL-17 [44], neutrophils may have potentials to initiate inflammasome activation and modulation of IL-33 leading to amplification of immune responses in asthmatic airways. Further researches are mandatory to solve the role of neutrophils in asthma, especially neutrophilic airway inflammation.
S100 protein family is a multigenic family of calcium modulated proteins that have a function of DAMP [57]. S100A9 belongs to the large Ca2-binding S100 protein family with the potential to form a complex with S100A8 (S100A8/A9: calprotectin), and exerts movement and activation of neutrophils [58]. It is one of the most abundant proteins found in neutrophils, macrophages, and epithelial cells during chronic inflammation. S100A8/A9 protein complex represents the exclusive arachidonic acid (AA)-binding proteins, which are important mediators of asthma, in human neutrophils [59]. AA derived from the complex could be used to generate other inflammatory mediators such as leukotriene B4, which trigger leukocyte activation and damage of vascular endothelium and smooth muscle cells, thereby exacerbating inflammation [60]. Recently, Kang et al. [61] found that S100A9 protein stimulates human bronchial epithelial cells to induce MUC5AC protein, which is the main mucin protein in asthmatic airways, via TLR4 activation. A previous proteomic study of ours [62] demonstrates increased levels of S100A9 in sputum of neutrophilic patients with uncontrolled severe asthma compared to those with controlled asthma (Fig. 2). Elevated S100A9 levels are limited to patients who are refractory to high doses of inhaled steroids; consequently, the function of S100A9 is suggested to relate with the resistance of airways to steroid treatment in severe asthma. In our study, S100A9 stimulates the activity of NF-κB and inflammasome activation (Fig. 3). IL-1β, a product of Inflammasome activation, also stimulates S100A9 induction by monocyte cell lines (Fig. 3). Thus, inflammasome stimulate inflammatory cells and epitheial cells to synthesize S100A9, which in turn could trigger activation of inflammasome by leukocyte leading to amplification of inflammation (Fig. 4).
Interestingly, S100A9 is revealed to promote proliferation of fibroblasts and up-regulate expression of collagen type III, α-smooth muscle actin and receptor for advanced glycation end-product [63]. Consequently, S100A9 may be a candidate that initiate and amplifies the neutrophilic inflammation of asthma as well as airway remodeling in collaboration with IL-17 (Fig. 4).
Neutrophilc inflammation of asthma has distinguished clinical features such as severe asthma and steroid resistance. This pattern may originate from the exaggerated activation of inflammasome, Th17 over-activation, and neutrophilic inflammation. In addition to caspase-1 activation, proteinase 3 and other protease from activated neutrophils directly cleave pro-IL-1β and pro-IL-18 to IL-1β and IL-18 that contribute to the phenotype of innate immune response. Neutrophilics in asthmatic airways may exert an additional effect of initiating inflammasome activation and amplifying immune responses. Among the several mediators from neutrophils, S100A9 seems to be one of candidate mediators to explain the action of neutrophils in amplifying neutrophilc inflammation. Further research in this area should focus on the potential for S100A9 in the induction of airway remodeling and steroid resistance, especially neutrophilic asthma.
Figures and Tables
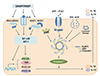 | Fig. 1A schematic overview of inflammasome activation. Extracellular and intracellular pathogen-associated molecular patterns (PAMPs) are recognized by Toll-like receptors (TLRs), which results in the transcription of pro-IL-1β and pro-IL-18 cytokines via induces nuclear factor (NF)-κB signaling. NOD-like receptor family, pyrin domain containing 3 activation (NLRP3) is induced by K+ efflux, reactive oxygen species (ROS) production, and activation of P2X7R by adenosine triphosphate (ATP). The assembly of the NLRP3 inflammasome complex, including the adaptor molecule apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC) and procaspase-1, leads to caspase-1 activation. The activated caspase-1 cleaves pro-IL-1β and pro-IL-18 into their mature forms. DAMP, danger-associated molecular pattern; NOD, nucleotide-binding oligomerization domain; IL, interleukin; RIG, retinoic acidinducible gene; MDA, melanoma differentiation-associated protein; INF, interferon; TNF, tumor-necrosis factor. |
 | Fig. 2(A) Photographs of 2-dimensional electrophoresis protein separation of pooled sputum (n = 5) obtained from uncontrolled asthmatics with neutrophilic inflammation (>70%). Protein spots are dentified by matrix-assisted laser desorption/ionizatione time of flight/time of flight. Enlargements of spots 2 is S100 calcium binding protein A9 (S100A9). (B) Comparison of S100A9 levels in sputum between the neutrophilic (neu) and eosinophilic (eos) subtypes of severe uncontrolled asthma (UA), partially controlled asthma (PA), and controlled asthma (CA) Using enzyme-linked immunosorbent assay. Data are presented as median (interquartile range). *p < 0.01 vs. neutrophilic subtypes of severe uncontrolled asthma. Adapted from Lee TH, et al. Ann Allergy Asthma Immunol 2013;111:268-275.e1, with permission of Elsevier B. V. [62]. |
 | Fig. 3(A) Interleukin-1β enhances S100 calcium binding protein A9 protein expression in cell extracts of THP-1 monocyte stimulated for 24 hours to 96 hours in the presence of CaCl2. (B) S100A9 dose - dependently induces inflammasome activation by cleaving procaspase-1 into P20 activated caspase-1 in the monocyte cell lines. |
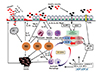 | Fig. 4Interaction of inflammasome activation and S100A9 in neutrophilic inflammation of asthma. Asthma is characterized by the airway infiltration of eosinophils and mast cells. IgE-dependent asthma is under the influence of the T helper 2 (Th2)-cytokines (interleukin [IL] 4, IL-5, and IL-13) generated by Th2-type cells. In T-cell independent manner, TSLP and IL-33 released from the activated epithelium stimulates mast cells to release the Th2-type effector cytokines. These can attract and activate eosinophils and mast cells. As another pathway, PAMPs and DAMPs including offending allergens induces nuclear factor (NF)-κB activation via Toll-like receptor (TLR) and concurrent inflammasome activation via nucleotide-binding oligomerization domain (NOD) receptors; subsequent activation of Th17 cells to regulate both neutrophilic and macrophage inflammation. TSLP, thymic stromal lymphopoietin, pathogen-associated molecular patterns; NLRP3, NOD-like receptor family, pyrin domain containing 3 activation; PAMP, damage-associated molecular patterns; DAMP, danger-associated molecular pattern; INF, interferon; TNF, tumor-necrosis factor. |
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2013R1A2A2A01068077).
References
1. Lecture on asthma-delivered at hotel Dieu, by Prof. Trousseau. Boston Med Surg J. 1859; 59:473–480.
2. Holgate ST. The 1992 cournand lecture. Asthma: past, present and future. Eur Respir J. 1993; 6:1507–1520.
3. Holgate ST. Mechanisms of asthma and implications for its prevention and treatment: a personal journey. Allergy Asthma Immunol Res. 2013; 5:343–347.

4. Ozato K, Shin DM, Chang TH, Morse HC 3rd. TRIM family proteins and their emerging roles in innate immunity. Nat Rev Immunol. 2008; 8:849–860.

5. Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006; 24:353–389.

6. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002; 10:417–426.
7. Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, Flavell RA, Sato V, Harding MW, Livingston DJ, Su MS. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997; 275:206–209.
8. Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U S A. 2009; 106:9021–9026.

9. Zhang Y, Xu CB, Cardell LO. Long-term exposure to IL-1beta enhances Toll-IL-1 receptor-mediated inflammatory signaling in murine airway hyperresponsiveness. Eur Cytokine Netw. 2009; 20:148–156.
10. Mahajan B, Vijayan VK, Agarwal MK, Bansal SK. Serum interleukin-1beta as a marker for differentiation of asthma and chronic obstructive pulmonary disease. Biomarkers. 2008; 13:713–727.
11. Broide DH, Lotz M, Cuomo AJ, Coburn DA, Federman EC, Wasserman SI. Cytokines in symptomatic asthma airways. J Allergy Clin Immunol. 1992; 89:958–967.

12. Sousa AR, Trigg CJ, Lane SJ, Hawksworth R, Nakhosteen JA, Poston RN, Lee TH. Effect of inhaled glucocorticoids on IL-1 beta and IL-1 receptor antagonist (IL-1 ra) expression in asthmatic bronchial epithelium. Thorax. 1997; 52:407–410.

13. Ando M, Shima M. Serum interleukins 12 and 18 and immunoglobulin E concentrations and allergic symptoms in Japanese schoolchildren. J Investig Allergol Clin Immunol. 2007; 17:14–19.
14. Patil SP, Wisnivesky JP, Busse PJ, Halm EA, Li XM. Detection of immunological biomarkers correlated with asthma control and quality of life measurements in sera from chronic asthmatic patients. Ann Allergy Asthma Immunol. 2011; 106:205–213.

15. Tanaka H, Miyazaki N, Oashi K, Teramoto S, Shiratori M, Hashimoto M, Ohmichi M, Abe S. IL-18 might reflect disease activity in mild and moderate asthma exacerbation. J Allergy Clin Immunol. 2001; 107:331–336.

16. Simpson JL, Phipps S, Baines KJ, Oreo KM, Gunawardhana L, Gibson PG. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur Respir J. 2014; 43:1067–1076.

17. Schroder NW. The role of innate immunity in the pathogenesis of asthma. Curr Opin Allergy Clin Immunol. 2009; 9:38–43.
18. Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009; 30:556–565.

19. Bauer RN, Brighton LE, Mueller L, Xiang Z, Rager JE, Fry RC, Peden DB, Jaspers I. Influenza enhances caspase-1 in bronchial epithelial cells from asthmatic volunteers and is associated with pathogenesis. J Allergy Clin Immunol. 2012; 130:958–967.e14.

20. Stout-Delgado HW, Vaughan SE, Shirali AC, Jaramillo RJ, Harrod KS. Impaired NLRP3 inflammasome function in elderly mice during influenza infection is rescued by treatment with nigericin. J Immunol. 2012; 188:2815–2824.

21. Triantafilou K, Kar S, van Kuppeveld FJ, Triantafilou M. Rhinovirus-induced calcium flux triggers NLRP3 and NLRC5 activation in bronchial cells. Am J Respir Cell Mol Biol. 2013; 49:923–934.

22. He X, Mekasha S, Mavrogiorgos N, Fitzgerald KA, Lien E, Ingalls RR. Inflammation and fibrosis during Chlamydia pneumoniae infection is regulated by IL-1 and the NLRP3/ASC inflammasome. J Immunol. 2010; 184:5743–5754.
23. Dickson RP, Erb-Downward JR, Huffnagle GB. The role of the bacterial microbiome in lung disease. Expert Rev Respir Med. 2013; 7:245–257.

24. Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, Moffatt MF, Cookson WO. Disordered microbial communities in asthmatic airways. PLoS One. 2010; 5:e8578.

25. Kool M, Petrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008; 181:3755–3759.

26. Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008; 453:1122–1126.

27. Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, Virchow JC Jr, Lambrecht BN. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007; 13:913–919.

28. Hirota JA, Hirota SA, Warner SM, Stefanowicz D, Shaheen F, Beck PL, Macdonald JA, Hackett TL, Sin DD, Van Eeden S, Knight DA. The airway epithelium nucleotide-binding domain and leucine-rich repeat protein 3 inflammasome is activated by urban particulate matter. J Allergy Clin Immunol. 2012; 129:1116–1125.e6.

29. Allen IC, Jania CM, Wilson JE, Tekeppe EM, Hua X, Brickey WJ, Kwan M, Koller BH, Tilley SL, Ting JP. Analysis of NLRP3 in the development of allergic airway disease in mice. J Immunol. 2012; 188:2884–2893.

30. Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009; 27:519–550.

31. Yilmaz O, Sater AA, Yao L, Koutouzis T, Pettengill M, Ojcius DM. ATP-dependent activation of an inflammasome in primary gingival epithelial cells infected by Porphyromonas gingivalis. Cell Microbiol. 2010; 12:188–198.
32. Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009; 30:576–587.

33. Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009; 31:331–341.
34. Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley SB, Menon S, Kastelein R, Bazan F, O'Garra A. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity. 1997; 7:571–581.
35. Hayashi N, Yoshimoto T, Izuhara K, Matsui K, Tanaka T, Nakanishi K. T helper 1 cells stimulated with ovalbumin and IL-18 induce airway hyperresponsiveness and lung fibrosis by IFN-gamma and IL-13 production. Proc Natl Acad Sci U S A. 2007; 104:14765–14770.
36. Besnard AG, Togbe D, Couillin I, Tan Z, Zheng SG, Erard F, Le Bert M, Quesniaux V, Ryffel B. Inflammasome-IL-1-Th17 response in allergic lung inflammation. J Mol Cell Biol. 2012; 4:3–10.

38. Robbins GR, Wen H, Ting JP. Inflammasomes and metabolic disorders: old genes in modern diseases. Mol Cell. 2014; 54:297–308.

39. Lamblin C, Gosset P, Tillie-Leblond I, Saulnier F, Marquette CH, Wallaert B, Tonnel AB. Bronchial neutrophilia in patients with noninfectious status asthmaticus. Am J Respir Crit Care Med. 1998; 157:394–402.

40. Jang AS, Kwon HS, Cho YS, Bae YJ, Kim TB, Park JS, Park SW, Uh ST, Choi JS, Kim YH, Hwang HK, Moon HB, Park CS. Identification of subtypes of refractory asthma in Korean patients by cluster analysis. Lung. 2013; 191:87–93.

41. Choi JS, Jang AS, Park JS, Park SW, Paik SH, Park JS, Uh ST, Kim YH, Park CS. Role of neutrophils in persistent airway obstruction due to refractory asthma. Respirology. 2012; 17:322–329.

42. Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, Wenzel SE, Peters SP, Meyers DA, Bleecker ER. National Heart, Lung, and Blood Institute's Severe Asthma Research Program. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014; 133:1557–1563.e5.

43. Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006; 7:135.

44. Ramirez-Velazquez C, Castillo EC, Guido-Bayardo L, Ortiz-Navarrete V. IL-17-producing peripheral blood CD177+ neutrophils increase in allergic asthmatic subjects. Allergy Asthma Clin Immunol. 2013; 9:23.

45. Sugawara S, Uehara A, Nochi T, Yamaguchi T, Ueda H, Sugiyama A, Hanzawa K, Kumagai K, Okamura H, Takada H. Neutrophil proteinase 3-mediated induction of bioactive IL-18 secretion by human oral epithelial cells. J Immunol. 2001; 167:6568–6575.

46. Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009; 15:410–416.

47. Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, Karp CL. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009; 457:585–588.

48. Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008; 8:193–204.

49. Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008; 9:310–318.

50. Kouzaki H, Iijima K, Kobayashi T, O'Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011; 186:4375–4387.

51. Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel 'alarmin'? PLoS One. 2008; 3:e3331.

52. Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, Paul W. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci U S A. 2009; 106:13463–13468.

53. Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010; 463:540–544.

54. Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005; 23:479–490.

55. Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G. Interleukin-33 is biologically active independently of caspase-1 cleavage. J Biol Chem. 2009; 284:19420–19426.

56. Bae S, Kang T, Hong J, Lee S, Choi J, Jhun H, Kwak A, Hong K, Kim E, Jo S, Kim S. Contradictory functions (activation/termination) of neutrophil proteinase 3 enzyme (PR3) in interleukin-33 biological activity. J Biol Chem. 2012; 287:8205–8213.

57. Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochem Biophys Res Commun. 2004; 322:1111–1122.

58. Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol. 2003; 170:3233–3242.

59. Kerkhoff C, Klempt M, Kaever V, Sorg C. The two calcium-binding proteins, S100A8 and S100A9, are involved in the metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1999; 274:32672–32679.

60. Kannan S. Inflammation: a novel mechanism for the transport of extracellular nucleotide-induced arachidonic acid by S100A8/A9 for transcellular metabolism. Cell Biol Int. 2003; 27:593–595.

61. Kang JH, Hwang SM, Chung IY. S100A8, S100A9, and S100A12 activate airway epithelial cells to produce MUC5AC via ERK and NF-κB pathways. Immunology. 2014; 06. 30. [Epub]. http://dx.doi.org/10.1111/imm.12352.
62. Lee TH, Jang AS, Park JS, Kim TH, Choi YS, Shin HR, Park SW, Uh ST, Choi JS, Kim YH, Kim Y, Kim S, Chung IY, Jeong SH, Park CS. Elevation of S100 calcium binding protein A9 in sputum of neutrophilic inflammation in severe uncontrolled asthma. Ann Allergy Asthma Immunol. 2013; 111:268–275.e1.

63. Xu X, Chen H, Zhu X, Ma Y, Liu Q, Xue Y, Chu H, Wu W, Wang J, Zou H. S100A9 promotes human lung fibroblast cells activation through receptor for advanced glycation end-product-mediated extracellular-regulated kinase 1/2, mitogen-activated protein-kinase and nuclear factor-κB-dependent pathways. Clin Exp Immunol. 2013; 173:523–535.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download