Abstract
Background
Allergic rhinitis and rhinosinusitis, common and debilitating conditions, should be managed in accordance with guideline recommendations. Guideline adherence shows regional differences. As of now, there is little data from Asia and none from Malaysia on the current treatment practices and unmet needs in the management of these conditions.
Objective
The objective of this study was to assess the current practice in the management of allergic rhinitis and rhinosinusitis by conducting a survey among ear, nose and throat (ENT) specialists, pharmacists, and general practitioners (GPs) in Malaysia.
Methods
We conducted a survey study among ENT specialists, pharmacists, and GPs in Malaysia, who answered a multiple choice questionnaire focused on the current practice in the management of allergic rhinitis and rhinosinusitis in their respective field. More than 200 ENT specialists, 100 pharmacists, and 200 GPs participated in the survey.
Results
Antihistamines were the most preferred choice for the treatment of mild allergic rhinitis by ENT specialists (45%), pharmacists (78%), and GPs (51%), with the most preferable duration of <2 weeks. In moderate-to-severe allergic rhinitis, a combination of antihistamines and intranasal steroids was the most preferred treatment of choice in 90% of ENT specialists, 72% of pharmacists, and 69% of GPs. Efficacy of antihistamines was the main criteria of choice in 58%, 53%, and 38% of ENT specialists, pharmacists, and GPs, respectively. Notably, complaints of drowsiness associated with nonsedative antihistamines were the major unmet need identified in the survey. For chronic rhinosinusitis, a combination of antihistamines and intranasal steroids was the most preferred treatment. The majority of the respondents preferred a treatment duration of >3 months with antihistamines. Satisfaction with the recommendations in the current Allergic Rhinitis and its Impact on Asthma (ARIA) guideline was high; 66%, 58%, and 89% of the ENT specialists, pharmacists, GPs, respectively, reported that the current ARIA guidelines are sufficient for their clinical/pharmacy practice.
Allergic rhinitis (AR) is a common disease, which has an increasing prevalence worldwide including in Asia [1]. In Europe, AR affects at least 21-23% of the population [2]. The Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines 2010 provide several recommendations for the management of AR [3]. However, many recent reports have indicated that there are still numerous unmet needs in the management of AR. A 2011 Asia-Pacific survey, for example, has shown that intranasal corticosteroids are associated with low rates of satisfaction as patients feel that they are inadequately effective and have bothersome side-effects [1]. Results of a survey involving America, Asia Pacific, Latin America, and Middle East showed that a low percentage of patients actually receive treatment with nasal sprays, which are considered the 'gold standard' of treatments for nasal allergies [4].
A Canadian survey identified the lack of persistent relief of AR symptoms as a major unmet need [5]. Moreover, this study suggested the need for better patient education as an avenue to improve AR treatment. It also suggested a relatively low level of awareness among physicians, especially those in general practice/family medicine. Also, the need for better therapies to improve management of AR was emphasized, as common comorbid conditions such as asthma, rhinosinusitis, nasal polyps, and sleep apnea formed an unrecognized portion of the total burden of the disease [5]. A recent European survey concluded that although effective treatment exists for AR, patients generally wait too long to seek medical advice and healthcare providers do not actively screen early for allergies [2]. Another survey conducted in Europe showed a poor correlation between patients and physicians in reporting of disease severity [6]. These survey results collectively indicate that numerous unmet needs in AR management exist, which vary with different geographical regions and countries.
To better understand the current treatment patterns and unmet needs in AR management in Malaysia, we formed an international expert panel and carried out a survey among ear, nose and throat (ENT) specialists, pharmacists and general practitioners (GPs). The objectives of the survey were to identify and characterize the prescribing patterns among medical professionals in the treatment of AR and to understand the unmet needs of patients in terms of side-effects. Moreover, the survey also aimed to clarify if there is a need to change the current ARIA guidelines to address current unmet needs in the management of AR.
The survey participants comprised three groups, i.e., ENT specialists, pharmacists and GPs. The survey was conducted separately for each group. The survey of the ENT specialists was carried out during the 4th Asian Paediatric Otorhinolaryngology Congress and 5th Malaysian International ORL-HNS Congress on May 16, 2013 at Kuala Lumpur. The pharmacists were surveyed during a pharmacists' partnership program held at Port Dickson, Malaysia on June 8, 2013. The GPs were surveyed during a workshop on primary care organized by Malaysian Association of Sports Medicine in Penang, Malaysia on July 6, 2013.
The questionnaires were developed considering various aspects of therapeutic management of AR, such as prescribing/dispensing of antihistamines, decongestants and intranasal steroids, duration and dose of the treatment in AR and chronic rhinosinusitis. The questionnaires consisted of 22-25 questions. The questionnaires for each of the three groups-ENT specialists, pharmacists, and GPs-were slightly modified taking into account the role played by the group in the healthcare system. The primary purpose of the questionnaires was to understand the current practices and to identify the unmet needs in the management of AR in Malaysia.
The respondents, primarily, were ENT specialists in private practice or in ENT departments of state or university hospitals. Excluding a few representatives from Indonesia and other Asia-Pacific countries, all other respondents were from Malaysia. Adult patients constituted the main patient group for most of the specialists. The clinical experience and additional profile details of the ENT specialists are given in Table 1.
Most of the respondents were from individual private retail pharmacy. Among the other respondents, there was an equal representation from chain retail pharmacy, medical center/private hospital and government hospital. Dispensing was the main practicing role for majority of the participating pharmacists. Pharmacists who are involved in patient counseling or those involved in purchasing and other administrative functions also participated in this survey. The clinical experience and additional profile details of the participating pharmacists are given in Table 1.
Majority of the GPs were from private or group practice. Most of the surveyed GPs examined more than seven patients with a complaint of nasal or skin allergy in a week. The clinical experiences and additional profile details of the GPs are summarized in Table 1.
Antihistamines and oral decongestants were the preferred treatments of ENT specialists, pharmacists, and GPs in Malaysia for allergy-associated conditions such as AR and rhinosinusitis-73%, 86%, and 90% of the surveyed ENT specialists, pharmacists and GPs, respectively, used antihistamines and 47%, 53%, and 84% of the ENT specialists, pharmacists and GPs, respectively, dispensed/prescribed oral decongestants for these conditions.
For the treatment of mild AR, antihistamines were the most preferred treatment of choice in all the groups (45%, 78%, and 51% of the ENT specialists, pharmacists,and GPs, respectively) (Fig. 1A). For moderate-to-severe AR, the majority of ENT specialists (90%), pharmacists (72%), and GPs (69%) preferred a combination of antihistamines and intranasal steroids as the treatment of choice (Fig. 1B).
For mild AR, the preferred treatment duration was <2 weeks for antihistamines in all the groups (27%, 51%, and 58% of the surveyed ENT specialists, pharmacists, and GPs, respectively) (Fig. 1C). In patients with moderate-to-severe AR, 49% of the ENT specialists preferred to use antihistamines for >3 months (Fig. 1D). Almost an equal proportion of respondents in the pharmacist group preferred a treatment duration of 2-4 weeks or >1 month (36% and 33%, respectively). Among the GPs, an equal proportion of respondents preferred 2-4 weeks (26%), >1 month (27%) and > 3 months (29%). For intranasal steroids in patients with moderate-to-severe AR, more than half of the ENT specialists (51%) preferred a treatment of duration of >9 months. On the other hand, the pharmacists and GPs (47% and 39%, respectively) preferred a treatment duration of 6 months in this patient population.
In selecting antihistamines, respondents in all the groups reported efficacy as the main criterion (58%, 53%, and 38% of the ENT specialists, pharmacists, and GPs, respectively). Efficacy was preferred over other criteria such as sedating effects, cost-effectiveness and good safety profile. The majority of respondents from all the groups had received complaints of drowsiness from patients who were on nonsedating antihistamines (59%, 64%, and 63% of the ENT specialists, pharmacists, and GPs, respectively) (Table 2). A majority of ENT specialists (76%) and GPs (53%) were not in favor of increasing the dose beyond the recommended dose when patients failed to respond to the recommended dose of antihistamines (Table 3). However, a majority of the pharmacists (77%) were in favor of increasing the dose. The respondents who were in favor of increasing the dose preferred to double the recommended daily dose of antihistamines (96%, 85%, and 87% of the ENT specialists, pharmacists, and GPs, respectively). More than half of the respondents in the ENT specialists group (57%) did not observe an increase in side-effects in the updosed patients. Similarly, in the pharmacist and GP groups, only 28% and 34%, respectively, observed increased side-effects in the updosed patients, while 72% and 66% of them did not make such an observation.
Sedation was the most common side-effect observed with an increase in the daily recommended dose of antihistamines by most of the respondents in all the three groups (53%, 69%, and 67% of the ENT specialists, pharmacists, and GPs, respectively) (Table 4). Similarly, all three groups (36%, 38%, and 43% of ENT specialists, pharmacists, and GPs, respectively) found negative impact on work or school performance to be the most common nonnasal complaint. Other important complaints included difficulty sleeping and feeling tired or general malaise.
In chronic rhinosinusitis, a combination of antihistamines and intranasal steroids was the preferred treatment of choice in all the three groups (53%, 79%, and 73% of the ENT specialists, pharmacists, and GPs, respectively). Similarly, respondents in all the three groups (52%, 48%, and 46% of the ENT specialists, pharmacists, and GPs, respectively) preferred antihistamine treatment duration of >3 months. The preferred duration of treatment for intranasal steroids in patients with chronic rhinosinusitisin ENT specialist group (61%) was >9 months, whereas GPs preferred a shorter treatment duration of 6 months.
Most ENT specialists (66%), pharmacists (58%), and GPs (89%) were satisfied with the recommendations in the current ARIA guidelines and felt that there are no changes required (Fig. 2).
This is the first survey to assess the current treatment patterns and unmet needs related to the management of AR and rhinosinusitis of ENT specialists, pharmacists, and GPs, based on their clinical experience and patient interaction, from a Malaysian perspective. Our study reveals a consensus between the treatment preferences of the three groups, which is largely in line with the ARIA guideline recommendations. The ARIA guidelines 2010 recommend the new-generation nonsedating oral antihistamines, which do not affect cytochrome P450, for the treatment of mild AR [7]. In accordance with these guidelines, a majority of ENT specialists, GPs, and pharmacists reported antihistamines as the preferred treatment option for mild AR. Similarly, for moderate-to-severe AR, all the three surveyed groups reported a combination of an intranasal steroid and anantihistamine as their treatment of choice. The ARIA guidelines recommend an antihistamine and/or a decongestant or an intranasal steroid in no preferred order for the management of intermittent moderate-to-severe AR. However, the 2010 update on the ARIA guidelines suggests that regular use of a combination of oral decongestant and an oral antihistamine should be avoided [3]. For the management of persistent moderate-to-severe AR, the guidelines recommend intranasal steroids over antihistamines [8].
In contrast, the ARIA guideline recommendation on the duration of AR treatment is less well adopted in Malaysia. Depending on the severity of symptoms, ARIA guidelines recommend reviewing the patient after 2-4 weeks of therapy. The therapy may be stepped up in case of failure or stepped down if there are signs of improvement. On improvement, the guidelines recommend continuing therapy for 1 month [8]. In contrast, respondents from all the three medical professional groups in this study preferred <2 weeks of antihistamine treatment duration for mild AR. With respect to the ARIA guideline recommendation on the duration of treatment of moderate-to-severe AR, there were differences in adherence between the three groups that is, ENT specialists, GPs, and pharmacists. The ENT specialists preferred therapy of more than 3 months, while the pharmacists preferred 2-4 weeks to more than a month. The treatment duration preferred by the pharmacists is thus in agreement with the ARIA guidelines, which recommend an initial treatment duration of 2-4 weeks and on review, continuation up to 1 month in case of improvement [8]. The preference for GPs ranged from 2 to 4 weeks to more than 3 months.
Interestingly, in Malaysia, most ENT specialists appear to not be in favor of increasing the antihistamine dose in patients who are not responding to the recommended daily dosage. Two of the surveyed ENT specialists favored updosing more than 7 times the dose (Table 3). However, it should be noted that updosing >7 times the dose is not recommended in the management of AR according to the ARIA guidelines [3]. Most of the pharmacists, on the other hand, prefer to increase the dose. The GPs seem to be equally divided on the issue, with half of them preferring to updose while the rest refraining from it.
Sedation was the most frequently encountered side-effect with updosing. Second-generation antihistamines are preferred over first-generation antihistamines due to the low risk of sedation associated with them [9]. Surprisingly, in this survey, a major unmet need identified is that a majority of ENT specialists, GPs, and pharmacists receive complaints of sedation from patients who are on nonsedating antihistamines. This finding, thus, warrants further studies to assess the prevalence of sedation in patients treated with nonsedating antihistamines.
Also, most ENT specialists, pharmacists, and GPs were satisfied with the recommendations in the current ARIA guidelines and felt there were no changes required. Nevertheless, this survey revealed that certain healthcare providers' preferences may vary from the ARIA guidelines. Thus, further in depth survey may divulge a need to revisit the ARIA guidelines in the Malaysian perspective.
A synopsis of guidelines on acute rhinosinusitis and chronic rhinosinusitis proposed by five major groups-European Position Paper on Rhinosinusitis and Nasal Polyps 2007 (EP3OS), Rhinosinusitis Initiative (RI), Joint Task Force on Practice Parameters (JTFPP), Clinical Practice Guideline: Adult Sinusitis (CPG:AS), and the British Society for Allergy and Clinical Immunology (BSACI)-was published in 2011. It was noted that the guidelines are not in complete agreement with respect to best clinical practices. Moreover, owing to a dearth in the number of controlled studies on chronic rhinosinusitis, an algorithm for the treatment of chronic rhinosinusitis is lacking. For mild chronic rhinosinusitis with or without nasal polyps, the EP3OS guidelines recommend treatment with intranasal corticosteroids. A follow-up at 3 months can be used to assess patient status. The JTFPP guidelines suggest a role for antihistamines in chronic rhinosinusitis when the underlying risk factor is allergic rhinosinusitis. The BSACI guidelines, which are relatively brief and largely correspond with the EP3OS guidelines, also recommend the addition of an oral antihistamine in allergic patients [10].
This survey gives additional insights into the preferences of healthcare providers in Malaysia with respect to the management of chronic rhinosinusitis. It was revealed in this survey that a combination of an antihistamine and an intranasal steroid was the most preferred treatment of choice across the three groups. Most of the respondents from all the three groups preferred antihistamine treatment duration of more than 3 months. Most of the ENT specialists and GPs, preferred intranasal steroid treatment duration ranging from 6 months to more than 9 months.
Further studies on the management of chronic rhinosinusitis in Malaysia are warranted. A better understanding of chronic rhinosinusitis pathophysiology and additional studies on the use of available therapeutic modalities are needed.
AR has a significant impact on the quality of life of the patients [2]. A previous survey carried out on the management of AR has emphasized to proactively reach out to the patients and to help them receive optimal treatments. In this survey, treatment choices for AR made by the ENT specialists, GPs, and the pharmacist were largely in line with the ARIA guidelines. However, the survey results revealed a major unmet need in the form of sedation experienced by patients who were prescribed nonsedating antihistamines. Further efforts are required to identify approaches to overcome the unmet needs, for example, by adapting the guidelines to regional specifics.
Figures and Tables
 | Fig. 1(A) Preferred treatment of choice for mild allergic rhinitis. (B) Preferred treatment of choice for moderate-to-severe allergic rhinitis. (C) Preferred duration of treatment for antihistamines in mild allergic rhinitis. (D) Preferred duration of treatment for antihistamines in moderate-to-severe allergic rhinitis. ENT, ear, nose, and throat; PRN, as needed. |
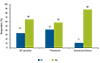 | Fig. 2Need for a change in Allergic Rhinitis and its Impact on Asthma guidelines. ENT, ear, nose, and throat. |
Table 2
Complaints of drowsiness received by respondents from patients who used nonsedating antihistamines

ACKNOWLEDGEMENTS
The Working Group was supported by an unrestricted educational grant from Merck Sharp & Dohme and Schering Plough (since merged). However, they had no other role in the content of this paper. Members of the Working Group contributed to and approved the content of this manuscript. BioQuest Solutions Pvt. Ltd. provided writing assistance and editorial support to the Working Group, and was funded by Merck Sharp & Dohme.
References
1. Katelaris CH, Lai CK, Rhee CS, Lee SH, Yun WD, Lim-Varona L, Quang VT, Hwang J, Singh H, Kim J, Boyle JM, Dhong HJ, Narayanan P, Vicente G, Blaiss M, Sacks R. Nasal allergies in the Asian-Pacific population: results from the Allergies in Asia-Pacific Survey. Am J Rhinol Allergy. 2011; 25:Suppl 1. S3–S15.

2. Maurer M, Zuberbier T. Undertreatment of rhinitis symptoms in Europe: findings from a cross-sectional questionnaire survey. Allergy. 2007; 62:1057–1063.

3. Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, van Wijk RG, Ohta K, Zuberbier T, Schunemann HJ. Global Allergy and Asthma European Network. Grading of Recommendations Assessment, Development and Evaluation Working Group. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010; 126:466–476.
4. Blaiss MS, Katelaris C, Neffen HE. Treatment of nasal allergies: results from the allergies surveys in America, Asia Pacific, Latin America, and Middle East. World Allergy Organ J. 2012; 5:Suppl 2. S146.
5. Keith PK, Desrosiers M, Laister T, Schellenberg RR, Waserman S. The burden of allergic rhinitis (AR) in Canada: perspectives of physicians and patients. Allergy Asthma Clin Immunol. 2012; 8:7.

6. Canonica GW, Bousquet J, Mullol J, Scadding GK, Virchow JC. A survey of the burden of allergic rhinitis in Europe. Allergy. 2007; 62:Suppl 85. 17–25.

7. Minor S. Allergic rhinitis: what's best for your patient? J Fam Pract. 2013; 62:E1–E10.
8. Mullol J. Positioning of antihistamines in the Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines. Clin Exp Allergy Rev. 2012; 12:17–26.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download