Abstract
Despite being one of the most common zoonotic infections worldwide, human toxocariasis has been one of the neglected tropical diseases. Although most human infections are asymptomatic, two main syndromes of human toxocariasis are classically recognized: systemic toxocariasis, which encompasses diseases in major organs; and ocular toxocariasis (OT), disease in the eye or optic nerve, caused by the migration of Toxocara larvae into the eye. OT is usually a unilateral disease, which typically presents as retinal granuloma, a yellowish or whitish inflammatory mass, in the posterior pole or peripheral retina. Granuloma itself or other comorbid conditions such as epiretinal membrane, macular edema, and retinal detachment can lead to permanent retinal damage and visual loss in eyes with OT. OT is diagnosed clinically by identification of clinical signs on ophthalmologic examination. Serological tests, such as enzyme-linked immunosorbent assay (ELISA) for detection of serum antibody against the Toxocara larvae, can confirm the diagnosis. In addition, serum immunoglobulin E and detection of ocular fluid antitoxocara antibody by ELISA may give additional aid to the diagnosis. Standard treatment of OT is corticosteroid in patients with active intraocular inflammation. Although the role of anthelmintic therapy is unclear, favorable outcome has been reported by combined corticosteroid and albendazole therapy in eyes with active inflammation. Prevention, by increasing public awareness and reducing the risk of infection, is also important. Recently, the association between ingestion of uncooked meat or liver and toxocariasis was reported, especially in adult patients. Future research on the potential source of infection, diagnosis, and treatment should be performed.
Toxocariasis is one of most common zoonotic infections worldwide mostly caused by Toxocara canis and less frequently by other roundworms such as Toxocara cati [1, 2]. Geographic distribution of toxocariasis is worldwide and seropositivity of toxocara antibody varies from 2.4% [3] to 76.6% [4]. Historically, in 1952, Beaver et al. [5] identified the etiologic agent, T. canis larvae, in eosinophilic granulomata in liver biopsies taken from three children. Four years later, Nichols [6] demonstrated the presence of the second-stage larvae of T. canis in histological sections of 24 eyes enucleated under suspicion of intraocular malignancies. These findings led to a common etiology for systemic and ocular diseases, human toxocariasis.
Individuals become infected with Toxocara when they unintentionally ingest embryonated eggs or larvae that have been shed in the feces of infected animals or uncooked paratenic hosts (Fig. 1) [1, 2, 7]. After a human ingests the eggs, infective larvae are released in the small intestine and subsequently, these penetrate the intestinal wall, enter the circulation, and migrate to organs where they induce inflammatory reactions and symptoms [1, 2]. Clinical spectrum of toxocariasis in humans varies from asymptomatic infection to severe organ injury, depending on the parasite load, the sites of larval migration, and the host's inflammatory response [1]. In particular, depending on the involved organ, two well-defined clinical syndromes can occur: systemic toxocariasis (also known as visceral larva migrans) and ocular toxocariasis (OT) [1].
OT is a clinically well-defined manifestation of intraocular infection by Toxocara larvae [1, 7]. OT affects both children and adults, with a mean age at onset ranging from 6.4 [8] to 51.7 [9] years in different studies. This is known to be an important cause of visual impairment during childhood [10]. Although human toxocariasis is one of the most common zoonotic infections worldwide, there are only a few reports that estimate the frequency of OT. For instance, the number of cases seen in eye clinics for vision loss in Alabama over a 6-month period was 11 cases per 1000 patients, and one case per 1,000 persons in the general population was estimated to have OT [11]. A study in Irish estimated the OT prevalence as 9.7 per 100,000 school children (4-19 years of age) [12]. In Asia, one Japanese epidemiologic survey showed that OT accounted for 1.1% of all uveitis cases [13]. In the past, most OT has been considered to develop in pediatric patients. However, recently, adult patients are predominantly affected by OT especially in Asians, which may be related to their food habit [9, 14, 15]. In Korea, systemic toxocariasis has been the major reason, accounting for 67-87%, of the high prevalence of eosinophilia (4.0-12.2%). In patients with systemic toxocariasis, about 60-90% had a history of raw cow liver ingestion [15]. Lack of knowledge and negligence leave many patients with toxocariasis abandoned and a part of the patients may suffer from OT.
As OT remains relatively unknown to the public as well as clinicians, the clinical features, diagnosis, treatment, and prevention of OT are reviewed here, with the focus on new developments in serologic diagnosis and novel findings from clinical studies in the literature.
The age at presentation in patients with OT may vary from one to 77 years of age [9, 16, 17]. Most of the previous studies reported that it is more common in males, as the male:female ratio was usually greater than 1:1 [8, 12, 18, 19, 20, 21, 22], up to 4.5:1 [23]. Most of the cases were unilateral and bilateral cases were less than 40% [20] in the literature. Its clinical presentations can be classified in one of the four forms: posterior pole granuloma, peripheral granuloma, nematode endophthalmitis, and atypical presentations [16].
Posterior pole granuloma (Fig. 2), a focal, whitish subretinal or intraretinal inflammatory mass usually less than 1 disc diameter with or without pigmentation, present in the posterior pole with or without signs of acute inflammation and hazy vitreous [16]. Wilkinson and Welch [24] showed that this form is the most common form of clinical presentation, consisting of 44% of OT cases. Macular lesions are most likely to be symptomatic and, hence, prompt patients to seek medical attention, which possibly explains the predilection of posterior pole granuloma.
Peripheral granuloma (Fig. 2), a focal, elevated, white nodule in the retinal periphery, may present with varying degrees of surrounding membranes and pigmentary changes [16]. In some of the patients with peripheral granuloma, inflammation may be diffuse and appear as a "snowbank" [10]. Fibrocellular bands may be observed running towards the posterior retina or optic nerve, sometimes forming a retinal fold. Localized traction on the retina may also result in tractional retinal detachment or rhegmatogenous retinal detachment by generating retinal holes or tears.
Nematode endophthalmitis is a type of panuveitis manifesting as a red, painful eye with diffuse intraocular inflammation [16]. Hypopyon and dense cellular infiltrate in vitreous can be observed in severe cases. Retinal granuloma may be observed through the vitreous haze as the vitreous opacity clears [24]; therefore, meticulous effort for the detection of retinal granuloma is important for the differential diagnosis. Patients with nematode endophthalmitis tend to be slightly younger than those with a localized granuloma.
Atypical presentations include inflammation and swelling of the optic nerve head (manifesting as optic neuritis), motile subretinal larvae, and diffuse chorioretinitis [1, 7, 10]. Anterior segment findings, such as conjunctivitis, keratitis, iridocyclitis, focal iris nodules, and cataract can also be observed [1]. In our recent report, small, round, white granuloma-like opacity moving in the subcapsular level of lens was observed in eyes with OT [25].
In addition to ocular inflammation and granuloma-associated presentations, comorbid conditions in eyes with OT require careful consideration as these can be other sources of vision loss and may progress if untreated. Such vitreoretinal comorbidities in OT include epiretinal membrane, vitreous opacity, tractional/rhegmatogenous retinal detachment, macular edema, cataract, and macular hole [9, 10]. Although typical presentation of OT, granuloma with intraocular inflammation, may be treated medically, cases with combined vitreoretinal comorbidities sometimes require surgical management for anatomic and visual recovery.
Several reports suggested causes of vision loss in patients with OT. Stewart et al. [10] reported that vitritis is the most common cause of vision loss in OT, followed by cystoid macular edema, tractional retinal detachment, and epiretinal membrane. Additionally, in eyes with macular granuloma, granuloma itself can lead to significant vision loss as it damages the involved retina and photoreceptors [9]. Thus, the causes of vision loss in eyes with OT can be grouped into 3 categories: retinal damage caused by granuloma itself, retinal comorbidities, and intraocular inflammation. In our case series of OT, the average best-corrected visual acuity was 20/64 Snellen equivalent at baseline, which was comparable to 20/56 Snellen equivalent at the final visit when intraocular inflammation was mostly subsided [9]. It indicates that in cases of visual decline, other causes, such as retinal damage by granuloma or other comorbid conditions should be considered and thoroughly evaluated during the clinical examination in patients with OT.
Remarkably, a unique feature of OT, compared to other inflammatory or retinal diseases, is intraocular migration (Fig. 3) [9, 26, 27]. Two case reports individually demonstrated intraocular migration of granuloma [26, 27]. There were two types of intraocular migration, continuous (granuloma migrated adjacent to the originally observed location) or discontinuous (a new granuloma far from the original location) [9]. During the clinical course, continuous and discontinuous granuloma migration was observed in 12.9% and 4.3% of eyes with OT, respectively [9]. As the migrating granuloma is pathognomonic for OT, this unique feature may be helpful in differentiating OT from other retinal diseases, such as ocular toxoplasmosis, sarcoidosis, tuberculosis, and fungal infections [9].
Definitive diagnosis of ocular toxocariasis can be obtained by histological demonstration of the toxocara larva or its fragments from biopsy of infected tissue. However, the collection of suitable biopsy material is risky and difficult in eyes with OT and rarely justified on clinical grounds. Thus, current diagnosis of OT is made clinically by the identification of the typical ophthalmologic signs and by the presence of serum antibody to the Toxocara larvae [1, 7, 16].
As mentioned above, clinical presentation of localized granuloma in the retinal posterior pole or periphery is typical for making the presumed diagnosis of OT. In cases of nematode endophthalmitis in which fundus examination is not possible due to vitreous opacity, specific ancillary tests such as ultrasonography (depiction of highly reflective mass with or without vitreous band) can be helpful for differential diagnosis and the presence of retinal granuloma should be re-evaluated for definite diagnosis when the vitreous becomes clear [1, 24].
Serologically, the standard, current test for diagnosing human toxocariasis is an indirect enzyme-linked immunosorbent assay (ELISA) based on the excretory-secretory antigens of T. canis. The ELISA (Biokema-Affinity Products, Crissier-Lausanne, Switzerland) [28] at a serum titer greater than 1: 32 has 78% sensitivity for the detection of toxocariasis [29] and Hagler et al. [30] suggested that a serum titer of 1: 8 is sufficient to support a diagnosis of ocular toxocariasis if the patient has signs and symptoms compatible with that disorder. In addition to the commercial ELISA kit, serodiagnosis by ELISA using crude antigen of toxocara larvae developed by researchers in Seoul National University in South Korea also showed acceptable diagnostic value with 92.2% sensitivity and 86.6% specificity [31]. It has been reported that recombinant antigens [32] can offer further solutions for serologic diagnosis by providing increased sensitivity and specificity compared to native excretory-secretory antigens. However, because of the inability of the parasite to mature into an adult form in humans, a search for T. canis or T. cati ova in human feces is unnecessary. Nationwide survey in the United States showed 17 of 25 patients (68%) with OT in which ELISA results were reported had positive test results [16]. However, the sensitivity and specificity of the ELISA for the diagnosis of OT has not been fully evaluated in large samples. Furthermore, unfortunately, such antibodies may often be undetectable or the titer may be below the cutoff level in the sera of OT patients [33], possibly due to the relatively low parasite loads in patients with OT. Therefore, the absence of serum antibodies does not rule out the diagnosis of OT. In this case, other ancillary tests can be helpful for confirming Toxocara infection.
As an ancillary test, the role for detection of immunoglobulin E (IgE) antibody has been identified in patients with human toxocariasis [7, 34]. Regarding the role in OT, our case series on OT showed 69.6% of clinically and serologically diagnosed patients showed elevated IgE levels, suggesting that IgE may provide a supplementary role for the diagnosis of OT [9]. Furthermore, levels of IgE showed a decrease after treatment in human toxocariasis, indicating that it may be useful for monitoring therapeutic effect. This necessitates further investigation on the role of IgE antibody among patients with OT.
Although systemic eosinophilia is an important feature of systemic toxocariasis [14, 35, 36, 37, 38], eosinophil count is not usually elevated in OT patients. For example, our case series showed only 11.6% (10 of 86) of patients with OT had eosinophilia [9]. Thus, eosinophil count may not be as helpful as the ELISA test or total IgE level; however, eosinophilia may indicate the possibility of co-occurring systemic and ocular toxocariasis [1, 34], which requires systemic evaluation and appropriate treatment.
It was suggested by several authors that improved sensitivity can be achieved using an ELISA analysis of intraocular fluids [17, 33, 39]. However, using the same cutoff value with serum antibody, the positive rates of ELISA on vitreous fluid was as low as 33% among the patients with OT, which requires further investigation on the suitable cutoff value for the detection of OT [9]. In surgically treated cases, remnants of Toxocara organisms have occasionally been detected from vitrectomy specimens obtained during surgery, which provide direct evidence of intraocular infection of Toxocara larva [40]. Cytologic examination of aqueous humor or vitreous samples may also be helpful in confirming the diagnosis of OT. However, currently no available data exist on the detection rate of vitreous cytology or biopsy among eyes with OT and thus, cytology and biopsy may be reserved for patients with suspected OT preplanned for vitreoretinal surgery.
Eyes with OT can be treated medically or surgically, depending on the severity of intraocular inflammation and comorbid conditions. First, medical therapy should be considered in cases of active inflammation. Current standard treatment for ocular toxocariasis is corticosteroid administration in patients with active intraocular inflammation. Topical and systemic corticosteroids are useful in managing intraocular inflammation and may reduce vitreous opacification and membrane formation [9, 24, 39, 41, 42].
The role of anthelmintic therapy in OT remains controversial as there have been no randomized controlled trials on the use of anthelmintic agents for OT. The results from only a few controlled trials of anthelmintic drugs for systemic toxocariasis have been published [43, 44]. Since parasitological cure cannot be assessed exactly, the outcome used in the published trials has simply been an improvement in the clinical signs and symptoms. Albendazole (400 mg given twice a day for 7-14 days) is the recommended standard drug for systemic toxocariasis and seems to be superior to thiabendazole (given at 50 mg/kg/day for 3-7 days) [43], which also strongly inhibits larval migration [45]. Diethylcarbamazine (given at 3-4 mg/kg/day for 21 days, starting at 25 mg/day for each adult patient and increasing the dose progressively) was also found to be effective for the treatment of systemic toxocariasis [44].
However, it is not proven that the anthelmintic therapy can kill intraocular Toxocara larvae as intraocular pharmacokinetic and pharmacodynamic studies on anthelmintic agents have not been performed. Therefore, the role of anthelmintic therapy in OT remains unclear. Nonetheless, the use of anthelmintic drugs combined with corticosteroids has shown favorable outcomes in many studies. For example, Barisani-Asenbauer et al. [41] reported that systemic albendazole (800 mg twice a day for adults and 400 mg twice a day for children) combined with steroid resulted in visual improvement without recurrences of uveitis in 5 patients throughout the 13.8-month observation period. In our study, combined corticosteroid and albendazole therapy significantly reduced 6-month recurrence (17.4%), as compared with the corticosteroid only group (54.5%), although the therapeutic improvement in inflammation and vision was similar with and without albendazole therapy [9]. The death of larva is considered to be associated with an inflammatory reaction, which has not been proven. However, albendazole monotherapy in OT patients with inactive intraocular inflammation showed no aggravation in intraocular inflammation [9]. Regarding the appropriate dosage of systemic albendazole therapy, there has been no consensus over clinicians. For instance, 200 mg twice a day for one month and 400 or 800 mg twice a day for 2 weeks were recommended for the dose of oral albendazole therapy for patients with OT, which have never been compared in a single study [9, 41].
A report in which motile subretinal larva destroyed with photocoagulation also exists [46]. In another report, intravitreal ranibizumab was shown effective for the treatment of choroidal neovascularization secondary to OT [47].
Medical therapy with systemic or topical corticosteroid is effective to reduce intraocular inflammation and improve inflammation-associated symptom but it has limited efficacy to resolve structural complications in the retina. Retinal detachment, epiretinal membrane, and persistent vitreous opacity are common surgical indications for vitreoretinal surgery performed in eyes with OT and several authors reported the outcome of the surgical treatment. Giuliari et al. [48] reported good anatomic and functional outcome of surgical treatment in 45 patients with OT. In our case series, 32 out of 101 patients (31.7%) required surgical treatment, each for epiretinal membrane (n = 19), vitreous opacity (n = 9), and/or retinal detachment (n = 2). Successful surgical outcome was achieved in 68.4%, 88.9%, and 50% of patients with epiretinal membrane, vitreous opacity, and retinal detachment, respectively, [9]. By providing structural modification, i.e., membrane peeling, removing vitreous opacification, or retinal reattachment, surgery in OT may result in stability or improvement in visual function.
Under the lack of sufficient awareness of OT, increasing public awareness on toxocariasis and reducing human exposure to Toxocara species are important for prevention of the disease. To reduce the risk of infection, source of transmission should be understood first.
Previous studies have established ownership of a dog or cat as a risk factor for infection with Toxocara. Direct contact with untreated, infected puppies has been considered to be an important source of transmission. However, nationwide survey performed in the United States showed percentages of pet ownership among OT patients were less than 50% (45% for dog and 26% for cat), suggesting that exposure to untreated, infected puppies may not sufficiently explain the sources of transmission. Accidental ingestion of embryonated eggs by geophagia was also suggested as another important source of transmission [1, 2, 7]. As the disease usually occurs in children, good hygiene practices, such as hand washing, especially after exposure to high-risk areas such as sandboxes, outdoor park, and playgrounds should be emphasized. Indeed, sandboxes, outdoor parks, and playgrounds can be highly contaminated with embryonated eggs of Toxocara as people routinely walk their pets at these places [2, 49, 50, 51, 52] and under warm conditions, embryonated eggs may remain viable for years [53]. Also, pet owners should be counseled to dispose pet feces promptly, to clean their pet's living area frequently, and to take their pets to the veterinarian for regular deworming [16].
In adult patients, source of transmission can be somewhat different to that in children as accidental ingestion of embryonated eggs is less likely to occur. The association between raw meat, especially raw cow liver, and toxocariasis has been recently reported in adult population [9, 14]. In some Asian countries, uncooked meat is consumed, mostly by adults, which may increase the number of adult patients with toxocariasis [15]. In Korea, a history of raw cow liver ingestion was found in 60-90% of systemic toxocariasis patients and in 80.8% of OT. The odds ratios of OT were 14.9 for raw cow liver ingestion and 2.28 for raw meat ingestion [9]. This indicates that the infection source of toxocariasis and demographic features of the patients may differ based on geographic and behavioral (especially, food habits) patterns. Public health practitioner should consider the local cultural context when identifying the probable infection sources in patients with toxocariasis and educate the people not to eat uncooked meat to prevent Toxocara infection.
Our current understandings of the diagnosis, treatment, and prevention of human toxocariasis and ocular involvement are limited, although it is one of the most common zoonotic infections worldwide. OT can be clinically diagnosed with specific signs. However, serologic diagnosis which provides evidence on Toxocara infection may greatly support the diagnosis in patients with presumed OT. Thus, further diagnostic improvement is necessary for better detection and prompt diagnosis of OT. In particular, future research should explore the potential sources of infection and standardize medical and surgical treatment for OT, to minimize anatomical and functional sequelae. Public education and campaign on preventing digestion of raw animal food, especially liver, may also reduce the morbidity of toxocariasis and OT.
Figures and Tables
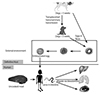 | Fig. 1A simplified figure showing the life cycle of Toxocara canis and its transmission route and migration in human. |
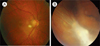 | Fig. 2Fundus photographs of retinal granuloma in a 67- (A) and 31-year-old male (B) patients with ocular toxocariasis. (A) Posterior pole granuloma appears as an oval, white lesion in the posterior pole of the retina. (B) Peripheral granuloma presents with an amorphous whitish mass with tractional membrane and retinal detachment. |
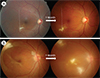 | Fig. 3Two migration patterns of Toxocara granuloma: continuous (A) and discontinuous (B). (A) Granuloma moves into the temporal side one month after the initial visit (A, left). The dotted line in panel A denotes a reference line connecting two reference points. (B) Compared to baseline, two novel granulomas appear in the macula and inferotemporal retina. |
References
1. Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol. 2010; 104:3–23.

2. Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003; 16:265–272.

3. Stensvold CR, Skov J, Moller LN, Jensen PM, Kapel CM, Petersen E, Nielsen HV. Seroprevalence of human toxocariasis in Denmark. Clin Vaccine Immunol. 2009; 16:1372–1373.

4. Fan CK, Hung CC, Du WY, Liao CW, Su KE. Seroepidemiology of Toxocara canis infection among mountain aboriginal schoolchildren living in contaminated districts in eastern Taiwan. Trop Med Int Health. 2004; 9:1312–1318.

5. Beaver PC, Snyder CH, Carrera GM, Dent JH, Lafferty JW. Chronic eosinophilia due to visceral larva migrans; report of three cases. Pediatrics. 1952; 9:7–19.
6. Nichols RL. The etiology of visceral larva migrans. I. Diagnostic morphology of infective second-stage Toxocara larvae. J Parasitol. 1956; 42(4 Section 1):349–362.

7. Smith H, Holland C, Taylor M, Magnaval JF, Schantz P, Maizels R. How common is human toxocariasis? Towards standardizing our knowledge. Trends Parasitol. 2009; 25:182–188.

8. Biglan AW, Glickman LT, Lobes LA Jr. Serum and vitreous Toxocara antibody in nematode endophthalmitis. Am J Ophthalmol. 1979; 88:898–901.

9. Ahn SJ, Woo SJ, Jin Y, Chang YS, Kim TW, Ahn J, Heo JW, Yu HG, Chung H, Park KH, Hong ST. Clinical features and course of ocular toxocariasis in adults. PLoS Negl Trop Dis. 2014; 8:e2938.

10. Stewart JM, Cubillan LD, Cunningham ET Jr. Prevalence, clinical features, and causes of vision loss among patients with ocular toxocariasis. Retina. 2005; 25:1005–1013.

11. Maetz HM, Kleinstein RN, Federico D, Wayne J. Estimated prevalence of ocular toxoplasmosis and toxocariasis in Alabama. J Infect Dis. 1987; 156:414.

12. Good B, Holland CV, Taylor MR, Larragy J, Moriarty P, O'Regan M. Ocular toxocariasis in schoolchildren. Clin Infect Dis. 2004; 39:173–178.

13. Goto H, Mochizuki M, Yamaki K, Kotake S, Usui M, Ohno S. Epidemiological survey of intraocular inflammation in Japan. Jpn J Ophthalmol. 2007; 51:41–44.

14. Choi D, Lim JH, Choi DC, Paik SW, Kim SH, Huh S. Toxocariasis and ingestion of raw cow liver in patients with eosinophilia. Korean J Parasitol. 2008; 46:139–143.

15. Lim JH. Foodborne eosinophilia due to visceral larva migrans: a disease abandoned. J Korean Med Sci. 2012; 27:1–2.

16. Woodhall D, Starr MC, Montgomery SP, Jones JL, Lum F, Read RW, Moorthy RS. Ocular toxocariasis: epidemiologic, anatomic, and therapeutic variations based on a survey of ophthalmic subspecialists. Ophthalmology. 2012; 119:1211–1217.

17. Alabiad CR, Albini TA, Santos CI, Davis JL. Ocular Toxocariasis in a Seronegative Adult. Ophthalmic Surg Lasers Imaging. 2010; 04. 02. 1–3. [Epub]. http://dx.doi.org/10.3928/15428877-20100325-06.

18. Yokoi K, Goto H, Sakai J, Usui M. Clinical features of ocular toxocariasis in Japan. Ocul Immunol Inflamm. 2003; 11:269–275.

19. Mirdha BR, Khokar SK. Ocular toxocariasis in a North Indian population. J Trop Pediatr. 2002; 48:328–330.

20. Park SP, Park I, Park HY, Lee SU, Huh S, Magnaval JF. Five cases of ocular toxocariasis confirmed by serology. Korean J Parasitol. 2000; 38:267–273.

21. Gillespie SH, Dinning WJ, Voller A, Crowcroft NS. The spectrum of ocular toxocariasis. Eye (Lond). 1993; 7(Pt 3):415–418.

22. Yoshida M, Shirao Y, Asai H, Nagase H, Nakamura H, Okazawa T, Kondo K, Takayanagi TH, Fujita K, Akao N. A retrospective study of ocular toxocariasis in Japan: correlation with antibody prevalence and ophthalmological findings of patients with uveitis. J Helminthol. 1999; 73:357–361.

23. Wan WL, Cano MR, Pince KJ, Green RL. Echographic characteristics of ocular toxocariasis. Ophthalmology. 1991; 98:28–32.

25. Ahn SJ, Woo SJ, Hyon JY, Park KH. Cataract formation associated with ocular toxocariasis. J Cataract Refract Surg. 2013; 39:830–835.

26. Sivaratnam D, Subrayan V, Ali NA. Transvitreal migration of a Toxocara larva resulting in a second chorioretinal granuloma. Jpn J Ophthalmol. 2008; 52:416–417.

27. Suzuki T, Joko T, Akao N, Ohashi Y. Following the migration of a Toxocara larva in the retina by optical coherence tomography and fluorescein angiography. Jpn J Ophthalmol. 2005; 49:159–161.

28. Jacquier P, Gottstein B, Stingelin Y, Eckert J. Immunodiagnosis of toxocarosis in humans: evaluation of a new enzyme-linked immunosorbent assay kit. J Clin Microbiol. 1991; 29:1831–1835.

30. Hagler WS, Pollard ZF, Jarrett WH, Donnelly EH. Results of surgery for ocular Toxocara canis. Ophthalmology. 1981; 88:1081–1086.

31. Jin Y, Shen C, Huh S, Sohn WM, Choi MH, Hong ST. Serodiagnosis of toxocariasis by ELISA using crude antigen of Toxocara canis larvae. Korean J Parasitol. 2013; 51:433–439.

32. Loukas A, Mullin NP, Tetteh KK, Moens L, Maizels RM. A novel C-type lectin secreted by a tissue-dwelling parasitic nematode. Curr Biol. 1999; 9:825–828.

33. Sharkey JA, McKay PS. Ocular toxocariasis in a patient with repeatedly negative ELISA titre to Toxocara canis. Br J Ophthalmol. 1993; 77:253–254.

34. Elefant GR, Shimizu SH, Sanchez MC, Jacob CM, Ferreira AW. A serological follow-up of toxocariasis patients after chemotherapy based on the detection of IgG, IgA, and IgE antibodies by enzyme-linked immunosorbent assay. J Clin Lab Anal. 2006; 20:164–172.

35. Seo M, Yoon SC. A seroepidemiological survey of toxocariasis among eosinophilia patients in Chungcheongnam-do. Korean J Parasitol. 2012; 50:249–251.

36. Kim YH, Huh S, Chung YB. Seroprevalence of toxocariasis among healthy people with eosinophilia. Korean J Parasitol. 2008; 46:29–32.

37. Kwon NH, Oh MJ, Lee SP, Lee BJ, Choi DC. The prevalence and diagnostic value of toxocariasis in unknown eosinophilia. Ann Hematol. 2006; 85:233–238.

38. Arias Irigoyen J, Senent Sanchez CJ. Toxocariasis: a cause of hyper IgE and eosinophilia. J Investig Allergol Clin Immunol. 1995; 5:232–234.
40. Maguire AM, Green WR, Michels RG, Erozan YS. Recovery of intraocular Toxocara canis by pars plana vitrectomy. Ophthalmology. 1990; 97:675–680.

41. Barisani-Asenbauer T, Maca SM, Hauff W, Kaminski SL, Domanovits H, Theyer I, Auer H. Treatment of ocular toxocariasis with albendazole. J Ocul Pharmacol Ther. 2001; 17:287–294.

43. Stürchler D, Schubarth P, Gualzata M, Gottstein B, Oettli A. Thiabendazole vs. albendazole in treatment of toxocariasis: a clinical trial. Ann Trop Med Parasitol. 1989; 83:473–478.
44. Magnaval JF. Comparative efficacy of diethylcarbamazine and mebendazole for the treatment of human toxocariasis. Parasitology. 1995; 110(Pt 5):529–533.

45. Abdel-Hameed AA. Effect of thiabendazole on the migration of Toxocara canis larvae in the mouse. J Parasitol. 1984; 70:226–231.

46. Zygulska-Machowa H, Ziobrowski S. A case of ocular toxocariasis treated by xenon photocoagulation. Klin Oczna. 1987; 89:213–214.
47. Lyall DA, Hutchison BM, Gaskell A, Varikkara M. Intravitreal Ranibizumab in the treatment of choroidal neovascularisation secondary to ocular toxocariasis in a 13-year-old boy. Eye (Lond). 2010; 24:1730–1731.

48. Giuliari GP, Ramirez G, Cortez RT. Surgical treatment of ocular toxocariasis: anatomic and functional results in 45 patients. Eur J Ophthalmol. 2011; 21:490–494.

49. Queiroz ML, Simonsen M, Paschoalotti MA, Chieffi PP. Frequency of soil contamination by Toxocara canis eggs in the south region of Sao Paulo municipality (SP, Brazil) in a 18 month period. Rev Inst Med Trop Sao Paulo. 2006; 48:317–319.
50. Castillo D, Paredes C, Zanartu C, Castillo G, Mercado R, Munoz V, Schenone H. Environmental contamination with Toxocara sp. eggs in public squares and parks from Santiago, Chile, 1999. Bol Chil Parasitol. 2000; 55:86–91.
51. Giacometti A, Cirioni O, Fortuna M, Osimani P, Antonicelli L, Del Prete MS, Riva A, D'Errico MM, Petrelli E, Scalise G. Environmental and serological evidence for the presence of toxocariasis in the urban area of Ancona, Italy. Eur J Epidemiol. 2000; 16:1023–1026.
52. Akdemir C. Visceral larva migrans among children in Kütahya (Turkey) and an evaluation of playgrounds for T. canis eggs. Turk J Pediatr. 2010; 52:158–162.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download