Abstract
Atopic dermatitis (AD) is a very common chronic disease that reportedly affects 10%-20% of the general population. The prevalence of AD appears to be steadily increasing, at least in developing countries. Two pathogenetic mechanisms have been mentioned. Traditionally immunological aberrations are thought to be a primary event in the initial development of AD ("inside-to-outside hypothesis"). Another hypothesis assumes that there is an intrinsic defect in epidermal barrier. Due to this barrier defect, allergens or irritants can easily penetrate the epidermal barrier, and induce immunologic reaction secondarily ("outside-to-inside hypothesis"). These days the epidermal barrier defect seems to gain more support as a primary event than immunological aberrations in the early changes of AD since the filaggrin mutation was reported in AD patients. Clinically AD initially affects face, and with age, flexural areas are typically involved. AD has many different clinical features. Diagnostic criteria for AD in each country may be a little different, although based on the criteria proposed by Hanifin and Rajka. AD can be controlled effectively with topical and/or systemic treatments and fortunately spontaneously disappears with age. However, in some cases very resistant to conventional therapies, additional treatments such as immunosuppressive agents are needed.
Atopic dermatitis (AD) starts usually in infancy, and takes a very chronic relapsing course. Clinically many different features are seen, and sometimes it is difficult to diagnose correctly. Diagnostic criteria for AD in each country may be a little different, although based on the criteria proposed by Hanifin and Rajka [1].
AD is very common and affects 10%-30% of the general population [2, 3]. The prevalence of AD appears to be steadily increasing, at least in developing countries. AD has a genetic background strongly influenced by environmental factors. In a recent breakthrough in the genetics of AD, a mutation of filaggrin (FLG) was found specifically in patients [4]. Pathogenetically this means the epidermal barrier defect hypothesis gains support as a primary event in the evolution of AD rather than immunological aberrations [2, 5]. For treatment, the correction of barrier defect is the first practical step before specific therapies such as topical corticosteroids (TCSs), and/or topical calcineurin inhibitors (TCIs).
AD is very common disease. However, it is impossible to know the exact prevalence of AD in each country. Prevalence data in each country is usually based on questionnaire studies like International study of Asthma and Allergies in Childhood (ISAAC) [6, 7]. ISAAC is very simple to administer, but based on individual answers, so the prevalence of AD is quite commonly overestimated. If well-trained physicians examine the skin directly, they can find easily that actual prevalence is lower than that reported by questionnaire study.
Is this condition increasing worldwide? Some studies show increases in the last 10-20 years [8, 9]. Other studies, especially in Norway [10] and Denmark [11], indicate that the prevalence is approaching a plateau in recent years [12]. A very recent report from Korea based on the direct observation of skin by dermatologists shows that the prevalence in preschool children was lower than the questionnaire-based estimate (9.2% vs. 19.1%) [3].
It is difficult to determine the real prevalence, but my estimation is that it is still increasing in many developing countries affecting over 10% of the population while reaching a plateau at around 20% in Western countries. In Korea it seems to be still increasing compared to the past [13]. About 10% of those of pediatric age are affected, decreasing with age to about 3% of the adult population suffering from AD, similar to Japan [14, 15].
Certainly genetic factors play an important role in the development of AD although the exact gene is not confirmed yet. Recently there was a real breakthrough in the genetic study of AD, and FLG mutation was found in many patients with AD [4].
Two pathogenetic mechanisms have been proposed [2] (Fig. 1). Traditionally immunological aberrations are thought to be a primary event in the initial development of AD [16]. In the early stage of AD, so-called Th2 immunity predominates leading to increased IL-4, IL-5, IL-13, and IgE, but when it becomes chronic, Th1 immunity also prevails. This immunologically abnormal reaction occurs first and then the epidermal inflammation follows subsequently leading to epidermal barrier defects ("inside-to-outside hypothesis").
The other school of thought assumes that there is an intrinsic defect in the epithelial cells, that is, a defect in epidermal barrier. Due to this barrier defect allergens or many irritant stimuli can easily penetrate the epidermal barrier, and induce immunologic reaction secondarily ("outside-to-inside hypothesis").
These days more emphasis is given to the barrier defect in the early changes in the evolution of AD since the FLG mutation was reported. One of the most important recent discoveries is of FLG mutation in a significant number of AD patients [4]. FLG is a very important protein not only to maintain the mechanical epidermal barrier by assembling keratin filaments but also to give the skin moisture by producing natural moisturizing factor through its breakdown products. The loss of FLG thus leads to epidermal barrier defect [17, 18]. However, FLG mutation was reported in different ethnicities, actually exists in about 30% of the AD patients [19-22], and cannot explain all the clinical symptoms observed [17].
Epidermal barrier defects can be measured more objectively and easily by checking transepidermal water loss or hydration status [23]. We can apply this phenomenon more practically to therapy than immunologic alterations. Maintaining skin hydration by supplementing the epidermal barrier defect is the most important part of AD therapy in terms of this barrier correction [24].
The skin of AD patients is frequently colonized with Staphylococcus aureus (S. aureus). S. aureus-derived superantigens may be involved in disease aggravation [25], and systemic antibiotics help reduce the severity of disease in selected cases.
Clinical features of AD are quite different from person to person. Sometimes it is quite difficult to reach a final diagnosis of AD. In 1980 Hanifin and Rajka [1] proposed an array of major and minor features of AD based on the history and clinical picture, and this is the basis for the diagnostic criteria in each country. There is no specific marker for AD and this means that AD is a kind of syndrome based on the various clinical manifestations.
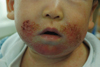
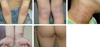
Clinically it usually starts around 2 months of age, and affects face and extensor surface of extremities typically in those below 2 years of age. These predilection sites are actually exposed areas prone to external irritation. The area around the mouth is typically affected due to irritation of saliva (Fig. 2), and sometimes periorbital area is also involved due to irritation of tears. With age over 2 years, flexural areas such as antecubital fossa, popliteal fossa, neck, ankle, wrist, and infragluteal folds are typically involved (Fig. 3). Skin dryness is usually severe even in those areas not showing eczematous features.
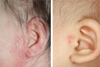
Sometimes it is difficult to make the final diagnosis of AD when patients show atypical clinical features. That is why we need diagnostic criteria for AD. Recently Korean diagnostic criteria for AD were reported [27] (Table 1) and are now under revision for more accurate diagnosis. These should be easy and suitable for field study in Korea. In fact there are many additional or minor clinical features such as scalp scaling, pityriasis alba, periauricular eczematization usually in the form of infraauricular and/or postauricular fissure, anterior neck folds, nipple eczema, palmar hyperlinearity, infragluteal eczema, and nonspecific hand-foot eczema. These features may be different according to ethnic background and age [28-31]. Periauricular eczematization (Fig. 4) is observed in around 60% of the Korean pediatric patients [32].
AD patients are prone to infections [33]. One of the explanations for the increased risk of cutaneous infections in AD is that the secretion of antimicrobial peptides such as defensins or cathelicidins that are important in innate immunity is decreased in AD [34, 35]. Eczematous skin lesions are frequently complicated by bacterial and viral infections. Bacterial infections presenting usually as folliculitis or furuncles are common. The skin manifestations of herpes simplex infection are often very severe involving large area of eczema lesions, so-called eczema herpeticum. Molluscum contagiosum are quite common, too.
In the adult type of AD in Korea, there are some differences from the childhood type. Onset age of AD seems to be a little later in adult type. The prevalence of respiratory atopy (asthma and allergic rhinitis) was higher and flexural dermatitis is more conspicuous in adult group [36]. Those adult patients with a much more severe form of AD refractory to the conventional therapies have increased recently.
Another classification of AD is that there are two forms, namely intrinsic and extrinsic [2, 37, 38]. Intrinsic form looks exactly same as the extrinsic form clinically, but lacks evidence for external sensitization. It shows neither elevated IgE, nor specific IgE to certain allergens. As expected, many of the infant patients have this intrinsic form, but they become sensitized to different allergens as they grow older, and become extrinsic patients.
AD is a disease that can be controlled effectively with topical and/or systemic treatments and fortunately spontaneously disappears with age. Only in some cases are they very resistant to therapies [39-43].
It is very important to educate patients to perform general measures such as avoiding aggravating factors and maintaining their skin clean and moist. Bathing hydrates skin, and removes possible allergens and irritants. Moisturizing immediately after bath further increases moisture contents of the skin, and can reduce the amount of topical steroids applied [24]. General recommendation for bathing is once daily with tepid water for approximately 10 to 20 minutes. Patients should avoid irritating materials including strong alkaline soaps, wool, and dirt. They should wear cotton clothes. Patients with AD quite commonly itch when they become anxious, frustrated or angry.
Allergic causation (food or aeroallergens) is frequently a concern of patients and especially of parents whose children have the disease. In many cases of AD with children, food may be an aggravating factor. However, it is not recommended that certain foods such as milk or eggs be absolutely avoided by all AD patients by simple conjectural correlation of those foods with the actual aggravation of their AD. AD can also be exacerbated by a wide variety of infections, including upper respiratory infections, herpes simplex as well as the very common S. aureus infections.
The mainstay treatment to reduce inflammation is still TCS. Generally weakest preparations are applied to face and genital areas and strongest ones are applied to hands and feet. Many patients have unwarranted steroid phobia and the proper use of TCS and patients education is utmost important. To reduce the use of TCS, we recommend patients that dryness of the skin be first managed with moisturizers and emollients as much as possible.
There are two topical forms, tacrolimus ointment and pimecrolimus cream. It effectively inhibits calcineurin (actually phosphatase) in T cells and mast cells, and blocks the synthesis of inflammatory cytokines from the immune cells. Both tacrolimus ointment (0.1% and 0.03%, Protopic®) and pimecrolimus 1% cream (Elidel®) are very effective especially in the face and neck lesions. Obviously, absorption into systemic circulations is minimal both in children and adults, and long-term safety is well established. They do not affect collagen synthesis, and do not cause skin atrophy contrary to TCS. That is why these can be applied safely and effectively to sensitive skin areas such as periorbital and intertriginous skin. Topical irritation in the form of burning, erythema or pruritus transiently felt after application is well tolerated.
Current practice is proactive treatment especially using tacrolimus ointment [44]. Proactive treatment means it is applied regularly 2-3 times a week even to the healthy looking areas of previously affected sites to prevent recurrences of AD.
Very recently it has been observed that topical use of TCIs would in time increase the risk of skin cancer, especially lymphoma, but these remain only theoretical possibilities that, to date, is lacking clinical evidence [45].
Pruritus that is refractory to moisturizers and conservative measures can be treated with antihistamines to a certain extent. There are controversies over the effect of antihistamines on atopic pruritus. Nevertheless, it is common practice for many doctors to prescribe antihistamines. Compared with the newer, non-sedating antihistamines, the older, sedating agents such as hydroxyzine seem to be more effective in controlling pruritus. However, these agents can affect a child's ability to learn or an adult's ability to drive. If drowsiness is a problem, a non-sedating antihistamine can be tried.
Antibiotics are used to treat secondary infections. Acute flare up of AD occurs frequently after infection and this usually can be relieved with a 5-7 day course of systemic antibiotic therapy such as first-generation cephalosporins and macrolide antibiotics. Secondarily infected crusted lesions are generally managed by soaking or wet-dressing the affected areas using cloths saturated with an aluminum acetate solution or a saline solution topically.
Systemic corticosteroids should be reserved for use in patients with severe treatment-resistant AD. Oral corticosteroids improve the lesions of AD, but a disease flare or rebound phenomenon usually occurs when systemic corticosteroids are stopped. This may lead to serious side effects of systemic corticosteroids in the end and should be discouraged.
Phototherapy is sometimes effective in treating refractory AD. It is generally recommended that UVA-1 be used in the initial phase of treatment to manage acute, severe exacerbations of AD and is followed by 311 nm narrow-band UVB (NB-UVB) therapy as means of maintenance therapy [46]. Currently NB-UVB therapy is usual practice worldwide [47].
Western literature reported that subcutaneous administration of interferon-gamma (IFN-γ) has proven effective in up to 80% of AD patients. However, these days it is rarely used in AD, because it is not so effective at least in chronic forms of AD. In chronic eczematous skin lesions of patients with AD, expression of Th1 cytokine IFN-γ also predominates. It seems that enthusiasm for the IFN-γ as a biologic response modifier for severe AD is decreasing.
This is a very useful drug in severe AD. It has great benefit especially for severe patients who have required long-term corticosteroid therapy. Clinical improvement is usually noted within a few weeks of starting therapy. Dose is usually initiated at 5 mg per kg per day in healthy patients with no renal or cardiovascular disease history. Laboratory studies including renal function test and liver function test should be checked biweekly at first and then at least on a monthly basis, along with weekly blood pressure checks early in the course of cyclosporine therapy. Among various immunosuppressants tried in AD cyclosporine is best characterized in terms of its dose schedule and side effects [48]. It does not increase the possibility of infection [49]. However, recurrence was usually noted within 2-4 weeks after stopping cyclosporine. Generally speaking, cyclosporine seems to be one of the most effective treatment options.
This immunosuppressive drug, once ingested, undergoes ester hydrolysis to its active form, mycophenolic acid (MPA). MPA blocks the proliferative responses of T and B lymphocytes. Oral mycophenolate mofetil is usually used at an initial dose of 1 g daily during the first week and 2 g daily for a further 11 weeks [50]. The most frequent side effect is GI trouble (nausea, vomiting, diarrhea) followed by hematologic effects (anemia, leukopenia, thrombocytopenia).
Methotrexate (MTX) is an antimetabolite (folate antagonist) and inhibits synthesis of inflammatory cytokine and chemotaxis of the immune cells. MTX is usually used as weekly regimen of three doses taken every 12 hours together with every day folate supplementation [53].
This drug is a purine analog with anti-proliferative and anti-inflammatory actions. A recent double blind placebo controlled study confirming its efficacy was reported in UK [54]. The main limitation of this is low onset of action, typically at least 4 weeks. Patients with deficient activity of thiopurine S-methyltransferase (TPMT) have increased risk for myelosuppression and the level of TPMT should be routinely tested before using this drug. In Korea, a routine checkup for TPMT is not available and this is not recommended.
These days sublingual immunotherapy (SLIT) seems to be more commonly used, usually targeted against house dust mite than subcutaneous immunotherapy (SCIT) [55]. SLIT or SCIT is reported to work effectively for respiratory atopy such as allergic rhinitis or asthma, but appears to have only limited value in AD. This kind of immunotherapy appears to be disappointing considering the complex procedures, high costs, and prolonged duration of therapy, at least 1-2 years [56].
It was reported that oral Lactobacillus GG supplementation given prenatally to mothers who had at least one first-degree relative with AD, allergic rhinitis or asthma, and postnatally for 6 months to their infants, reduces the risk of AD [57, 58]. However, there are currently insufficient data to recommend probiotics as standard therapy in AD.
Figures and Tables
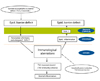 | Fig. 1Two pathogenetic mechanisms. Which one is first, immunological aberrations (inside-to-outside hypothesis) or epidermal barrier defect (outside-to-inside hypothesis)? These days more emphasis is given to barrier defect in the early changes in the evolution of AD ever since FLG mutation was reported.
FLG, filaggrin; Epid, epidermis; TEWL, transepidermal water loss; AD, atopic dermatitis.
|
References
1. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980. 59:44–47.
3. Choi WJ, Ko JY, Kim JW, Lee KH, Park CW, Kim KH, Kim MN, Lee AY, Cho SH, Park YL, Choi JH, Seo SJ, Lee YW, Roh JY, Park YM, Kim DJ, Ro YS. Prevalence and risk factors for atopic dermatitis: a cross-sectional study of 6,453 Korean preschool children. Acta Derm Venereol. 2012. 92:467–471.

4. Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, Goudie DR, Sandilands A, Campbell LE, Smith FJ, O'Regan GM, Watson RM, Cecil JE, Bale SJ, Compton JG, DiGiovanna JJ, Fleckman P, Lewis-Jones S, Arseculeratne G, Sergeant A, Munro CS, El Houate B, McElreavey K, Halkjaer LB, Bisgaard H, Mukhopadhyay S, McLean WH. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006. 38:441–446.

5. Cork MJ, Danby SG, Vasilopoulos Y, Hadgraft J, Lane ME, Moustafa M, Guy RH, Macgowan AL, Tazi-Ahnini R, Ward SJ. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. 2009. 129:1892–1908.

6. Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, Mitchell EA, Pearce N, Sibbald B, Stewart AW, Strachan D, Weiland SK, Williams HC. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995. 8:483–491.

7. Asher MI, Weiland SK. The International Study of Asthma and Allergies in Childhood (ISAAC). ISAAC Steering Committee. Clin Exp Allergy. 1998. 28:Suppl 5. 52–66.
8. Maziak W, Behrens T, Brasky TM, Duhme H, Rzehak P, Weiland SK, Keil U. Are asthma and allergies in children and adolescents increasing? Results from ISAAC phase I and phase III surveys in Munster, Germany. Allergy. 2003. 58:572–579.

9. Grize L, Gassner M, Wuthrich B, Bringolf-Isler B, Takken-Sahli K, Sennhauser FH, Stricker T, Eigenmann PA, Braun-Fahrlander C. Swiss Surveillance Programme on Childhood Allergy and Respiratory symptoms with respect to Air Pollution (SCARPOL) team. Trends in prevalence of asthma, allergic rhinitis and atopic dermatitis in 5-7-year old Swiss children from 1992 to 2001. Allergy. 2006. 61:556–562.

10. Selnes A, Nystad W, Bolle R, Lund E. Diverging prevalence trends of atopic disorders in Norwegian children. Results from three cross-sectional studies. Allergy. 2005. 60:894–899.

11. Olesen AB, Bang K, Juul S, Thestrup-Pedersen K. Stable incidence of atopic dermatitis among children in Denmark during the 1990s. Acta Derm Venereol. 2005. 85:244–247.

12. Yura A, Shimizu T. Trends in the prevalence of atopic dermatitis in school children: longitudinal study in Osaka Prefecture, Japan, from 1985 to 1997. Br J Dermatol. 2001. 145:966–973.

13. Oh JW, Pyun BY, Choung JT, Ahn KM, Kim CH, Song SW, Son JA, Lee SY, Lee SI. Epidemiological change of atopic dermatitis and food allergy in school-aged children in Korea between 1995 and 2000. J Korean Med Sci. 2004. 19:716–723.

14. Muto T, Hsieh SD, Sakurai Y, Yoshinaga H, Suto H, Okumura K, Ogawa H. Prevalence of atopic dermatitis in Japanese adults. Br J Dermatol. 2003. 148:117–121.

15. Saeki H, Iizuka H, Mori Y, Akasaka T, Takagi H, Kitajima Y, Tezuka T, Tanaka T, Hide M, Yamamoto S, Hirose Y, Kodama H, Urabe K, Furue M, Kasagi F, Torii H, Nakamura K, Morita E, Tsunemi Y, Tamaki K. Prevalence of atopic dermatitis in Japanese elementary schoolchildren. Br J Dermatol. 2005. 152:110–114.

16. Werfel T. The role of leukocytes, keratinocytes, and allergen-specific IgE in the development of atopic dermatitis. J Invest Dermatol. 2009. 129:1878–1891.

17. Brown SJ, Irvine AD. Atopic eczema and the filaggrin story. Semin Cutan Med Surg. 2008. 27:128–137.

18. Kubo A, Nagao K, Amagai M. Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases. J Clin Invest. 2012. 122:440–447.

19. Nomura T, Sandilands A, Akiyama M, Liao H, Evans AT, Sakai K, Ota M, Sugiura H, Yamamoto K, Sato H, Palmer CN, Smith FJ, McLean WH, Shimizu H. Unique mutations in the filaggrin gene in Japanese patients with ichthyosis vulgaris and atopic dermatitis. J Allergy Clin Immunol. 2007. 119:434–440.
20. Hsu CK, Akiyama M, Nemoto-Hasebe I, Nomura T, Sandilands A, Chao SC, Lee JY, Sheu HM, McLean WH, Shimizu H. Analysis of Taiwanese ichthyosis vulgaris families further demonstrates differences in FLG mutations between European and Asian populations. Br J Dermatol. 2009. 161:448–451.
21. Kang TW, Lee JS, Oh SW, Kim SC. Filaggrin mutation c.3321delA in a Korean patient with ichthyosis vulgaris and atopic dermatitis. Dermatology. 2009. 218:186–187.

22. Seo S, Park M, Jeong M, Kim J, Kim I, Li K. Clinical characteristics correlates with impaired barrier in filaggrin gene related Korean atopic dermatitis patients [abstract]. J Invest Dermatol. 2012. 132:S91.
23. Seidenari S, Giusti G. Objective assessment of the skin of children affected by atopic dermatitis: a study of pH, capacitance and TEWL in eczematous and clinically uninvolved skin. Acta Derm Venereol. 1995. 75:429–433.
24. Anderson PC, Dinulos JG. Are the new moisturizers more effective? Curr Opin Pediatr. 2009. 21:486–490.

25. Bunikowski R, Mielke M, Skarabis H, Herz U, Bergmann RL, Wahn U, Renz H. Prevalence and role of serum IgE antibodies to the Staphylococcus aureus-derived superantigens SEA and SEB in children with atopic dermatitis. J Allergy Clin Immunol. 1999. 103:119–124.

26. Schmid-Grendelmeier P, Flückiger S, Disch R, Trautmann A, Wüthrich B, Blaser K, Scheynius A, Crameri R. IgE-mediated and T cell-mediated autoimmunity against manganese superoxide dismutase in atopic dermatitis. J Allergy Clin Immunol. 2005. 115:1068–1075.
27. Park YL, Kim HD, Kim KH, Kim MN, Kim JW, Ro YS, Park CW, Lee KH, Lee AY, Cho SH, Choi JH. Report from ADRG: a study on the diagnostic criteria of Korean atopic dermatitis. Korean J Dermatol. 2006. 44:659–663.
28. Mevorah B, Frenk E, Wietlisbach V, Carrel CF. Minor clinical features of atopic dermatitis. Evaluation of their diagnostic significance. Dermatologica. 1988. 177:360–364.
29. Kanwar AJ, Dhar S, Kaur S. Evaluation of minor clinical features of atopic dermatitis. Pediatr Dermatol. 1991. 8:114–116.

30. Nagaraja , Kanwar AJ, Dhar S, Singh S. Frequency and significance of minor clinical features in various age-related subgroups of atopic dermatitis in children. Pediatr Dermatol. 1996. 13:10–13.

31. Lee HJ, Cho SH, Ha SJ, Ahn WK, Park YM, Byun DG, Kim JW. Minor cutaneous features of atopic dermatitis in South Korea. Int J Dermatol. 2000. 39:337–342.

32. Kim KH, Hwang JH, Park KC. Periauricular eczematization in childhood atopic dermatitis. Pediatr Dermatol. 1996. 13:278–280.

33. Hayashida S, Furusho N, Uchi H, Miyazaki S, Eiraku K, Gondo C, Tsuji G, Hachisuka J, Fukagawa S, Kido M, Nakahara T, Moroi Y, Hayashi J, Hagihara A, Furue M. Are lifetime prevalence of impetigo, molluscum and herpes infection really increased in children having atopic dermatitis? J Dermatol Sci. 2010. 60:173–178.

34. Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002. 347:1151–1160.

35. Hata TR, Gallo RL. Antimicrobial peptides, skin infections, and atopic dermatitis. Semin Cutan Med Surg. 2008. 27:144–150.

36. Kim KH, Park KC. Clinical characteristics of adult atopic dermatitis. Ann Dermatol. 1998. 10:229–232.

37. Seidenari S, Giusti F. Intrinsic vs. extrinsic atopic dermatitis: a study on diagnostic definition and prevalence [abstract]. J Invest Dermatol. 2003. 121:1255.
39. Krakowski AC, Dohil MA. Topical therapy in pediatric atopic dermatitis. Semin Cutan Med Surg. 2008. 27:161–167.

40. Borchard KL, Orchard D. Systemic therapy of paediatric atopic dermatitis: an update. Australas J Dermatol. 2008. 49:123–136.

41. Schmitt J, Schäkel K, Schmitt N, Meurer M. Systemic treatment of severe atopic eczema: a systematic review. Acta Derm Venereol. 2007. 87:100–111.

42. Darsow U, Wollenberg A, Simon D, Taieb A, Werfel T, Oranje A, Gelmetti C, Svensson A, Deleuran M, Calza AM, Giusti F, Lubbe J, Seidenari S, Ring J. European Task Force on Atopic Dermatitis/EADV Eczema Task Force. ETFAD/EADV eczema task force 2009 position paper on diagnosis and treatment of atopic dermatitis. J Eur Acad Dermatol Venereol. 2010. 24:317–328.

43. Saeki H, Furue M, Furukawa F, Hide M, Ohtsuki M, Katayama I, Sasaki R, Suto H, Takehara K. Committee for Guidelines for the Management of Atopic Dermatitis of Japanese Dermatological Association. Guidelines for management of atopic dermatitis. J Dermatol. 2009. 36:563–577.

44. Wollenberg A, Reitamo S, Girolomoni G, Lahfa M, Ruzicka T, Healy E, Giannetti A, Bieber T, Vyas J, Deleuran M. European Tacrolimus Ointment Study Group. Proactive treatment of atopic dermatitis in adults with 0.1% tacrolimus ointment. Allergy. 2008. 63:742–750.

45. Thaçi D, Salgo R. Malignancy concerns of topical calcineurin inhibitors for atopic dermatitis: facts and controversies. Clin Dermatol. 2010. 28:52–56.

47. Clayton TH, Clark SM, Turner D, Goulden V. The treatment of severe atopic dermatitis in childhood with narrowband ultraviolet B phototherapy. Clin Exp Dermatol. 2007. 32:28–33.

48. Hijnen DJ, ten Berge O, Timmer-de Mik L, Bruijnzeel-Koomen CA, de Bruin-Weller MS. Efficacy and safety of long-term treatment with cyclosporin A for atopic dermatitis. J Eur Acad Dermatol Venereol. 2007. 21:85–89.

49. Kim SW, Park YW, Kwon IH, Kim KH. Cyclosporin treatment of atopic dermatitis: is it really associated with infectious diseases? Ann Dermatol. 2010. 22:170–172.

50. Heller M, Shin HT, Orlow SJ, Schaffer JV. Mycophenolate mofetil for severe childhood atopic dermatitis: experience in 14 patients. Br J Dermatol. 2007. 157:127–132.

51. Kwon HH, Kim KH. Intravenous immunoglobulin treatment in a child with resistant atopic dermatitis. Ann Dermatol. 2012. 24:66–69.

52. Oh J, Kim J, Baik H, Lee H. Long-term efficacy of intravenous immunoglobulin therapy for moderate to severe atopic dermatitis in childhood. J Allergy Clin Immunol. 2011. 127:Ab36.

53. Lyakhovitsky A, Barzilai A, Heyman R, Baum S, Amichai B, Solomon M, Shpiro D, Trau H. Low-dose methotrexate treatment for moderate-to-severe atopic dermatitis in adults. J Eur Acad Dermatol Venereol. 2010. 24:43–49.

54. Berth-Jones J, Takwale A, Tan E, Barclay G, Agarwal S, Ahmed I, Hotchkiss K, Graham-Brown RA. Azathioprine in severe adult atopic dermatitis: a double-blind, placebo-controlled, crossover trial. Br J Dermatol. 2002. 147:324–330.

55. Cadario G, Galluccio AG, Pezza M, Appino A, Milani M, Pecora S, Mastrandrea F. Sublingual immunotherapy efficacy in patients with atopic dermatitis and house dust mites sensitivity: a prospective pilot study. Curr Med Res Opin. 2007. 23:2503–2506.

56. Compalati E, Rogkakou A, Passalacqua G, Canonica GW. Evidences of efficacy of allergen immunotherapy in atopic dermatitis: an updated review. Curr Opin Allergy Clin Immunol. 2012. 12:427–433.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download