Abstract
A diagnosis of food allergies should be made based on the observation of allergic symptoms following the intake of suspected foods and the presence of allergen-specific IgE antibodies. The oral food challenge (OFC) test is the most reliable clinical procedure for diagnosing food allergies. Specific IgE testing of allergen components as well as classical crude allergen extracts helps to make a more specific diagnosis of food allergies. The Japanese Society of Pediatric Allergy and Clinical Immunology issued the 'Japanese Pediatric Guideline for Food Allergy 2012' to provide information regarding the standardized diagnosis and management of food allergies. This review summarizes recent progress in the diagnosis of food allergies, focusing on the use of specific IgE tests and the OFC procedure in accordance with the Japanese guidelines.
Food allergies affect 5-10% of infants, 2-5% of toddlers and 1-2% of school children in Japan [1]. These allergies are associated with large social burdens, particularly in terms of providing school lunches to affected children [2] and preparing for unexpected severe reactions after accidental ingestion of allergic foods [3].
Laboratory testing to detect allergen-specific immunoglobulin E (sIgE) antibodies (ImmunoCAP®; Phadia KK, Japan) is widely used to diagnose food allergies in Japanese pediatric practice. The crude extracts of allergens are generally used in sIgE tests; however, testing for sIgE to the allergen components helps to make a more specific diagnosis [4].
The presence of sIgE offers proof of sensitization; however, it is not enough to make a diagnosis of food allergies without observing clinical manifestations following the ingestion of the offending food [5]. Examinations for sIgE are sometimes performed before introducing solid foods to atopic or eczematous babies. Transient elimination of sensitized foods may help to control allergic conditions in infants; however, a proper diagnosis of food allergies should be made after one year of age [6].
The occurrence of allergic symptoms following consumption of offending foods can be proven based on a convincing clinical history, although oral food challenge (OFC) testing provides the most reliable confirmation of symptoms. OFC testing has been covered by public health insurance in Japan since 2006. The Japanese Society of Pediatric Allergy and Clinical Immunology (JSPACI) issued the 'Japanese Pediatric Guideline for Oral Food Challenge Test in Food Allergy 2009' (Japanese OFC guidelines, available only in Japanese) to provide a safe and standardized method of administering OFC tests [7, 8]. Following this, an increasing number of pediatric institutes, including not only allergy specialists, but also general pediatric doctors, have begun to perform OFC testing in Japan. The Japanese Society of Food Allergy provides a site map of institutes in which OFC testing is available. According to the database (http://www.foodallergy.jp/), more than 100 hospitals currently perform more than 50 OFC tests each year (Table 1).
The JSPACI issued the Japanese Pediatric Guideline for Food Allergy 2012 (JPGFA 2012) [9] to reflect updated understanding and a standardized strategy for the diagnosis and management of food allergies. This review focuses on the diagnosis of food allergies, primarily based on the JPGFA 2012 guidelines.
Specific IgE testing is not the definitive diagnostic marker of food allergies; however, the titers of IgE indicate the likelihood or 'probability' of a true food allergy. The probability curve is the product of a logistic regression analysis of the sIgE titers calculated in accordance with the results of OFC testing [10]. The limitation of the probability curve, however, is that the sIgE titer hardly predicts the threshold dose of allergens or the severity of symptoms. The diagnostic power, in terms of sensitivity and specificity, varies between different allergens, and the sIgE titer must be evaluated based on appropriate knowledge of the allergen.
Hen's eggs are the most common food allergen in Japanese children. Hen's egg allergens, particularly ovalbumin (Gal d 2), are sensitive to denaturing by heat, resulting in the loss of IgE-binding capacity. Ovomucoid (Gal d 1), on the other hand, is resistant to heat and protease digestion [11]. As a result, an elevated sIgE titer to egg whites is a good marker of an unheated egg allergy, whereas an elevated sIgE titer to ovomucoid offers a good diagnostic marker of a heated egg allergy [12].
The probability curve for egg whites is well known and widely used in Japan [13] (Fig. 1A). However, it was created based on the OFC results of many patients with a past history of egg allergies. We previously reported new probability curves of sIgE to egg whites and ovomucoid, exclusively based on the OFC results of 1-year-old children who had never eaten any egg products, thereby representing the initial diagnosis of egg allergies [14]. The probability of egg allergies in our study was generally lower than that reported in a previous study, and the presence of an sIgE reaction to ovomucoid exhibited a higher probability than that to egg whites (Fig. 2).
A probability curve for the sIgE reaction to milk is also available for the diagnosis of milk allergies. This curve exhibits more than 95% positive predictive value (PPV) at 57.3 kUA/L (Fig. 1B). Caseins (Bos d 8) constitute 76-86% of whole milk proteins and are the major milk allergens. Receiver operating characteristic analyses of milk components demonstrate the advantage of using casein-specific IgE testing [15]; however, tests for other milk allergens, such as β-lactoglobulin and α-lactalbumin, are not sufficient to make a diagnosis of milk allergies (Fig. 3).
Wheat allergens can be divided into two types: water/salt soluble compounds (albumins and globulins) and gluten (gliadin and glutenin). Wheat and other cereal grains share a number of homologous proteins, mostly water/salt soluble compounds [16], whereas gluten is a component exclusive to wheat. The fact that most patients with wheat allergies can consume other cereals such as rice and corn suggests that the dominant wheat allergens and IgE epitopes exist in components that are not cross-reactive with other cereals.
sIgE testing to wheat uses water soluble components of wheat proteins. Therefore, the specificity of testing sIgE to wheat is limited, exhibiting less than 80% PPV, even at the highest titer (Fig. 4A). Specific IgE testing for recombinant ω-5 gliadin offers a good marker of immediate-type wheat allergies or anaphylaxis in children [17], as well as wheat-dependent exercise-induced anaphylaxis in adults [18]. The probability curve of sIgE to ω-5 gliadin exhibits more than 95% PPV at 3.5 kUA/L. On the other hand, the sensitivity of sIgE to ω-5 gliadin is limited to 80% at >0.35 kUA/L [19] because other allergenic components can contribute to allergic reactions (Fig. 4B).
Crude peanut extract contains many cross-reactive components to other plant-derived allergens such as profilin and cross-reactive carbohydrate determinants [20]. These components contribute to the clinically false-positive detection of sIgE antibodies to peanuts.
The allergen components of peanuts have been extensively characterized. The storage proteins Ara h 1 (7S albumin) and Ara h 2 (2S albumin) are known to be the major peanut allergens that cause anaphylaxis. Detecting sIgE to recombinant Ara h 2 indicates an 88% chance of having a peanut allergy. In combination with sIgE to Ara h 1, the possibility of a peanut allergy reaches almost 100% [21].
A well-documented clinical history of the occurrence of allergic symptoms following the consumption of an offending food can provide definitive information for the diagnosis of food allergies. Most mothers can clearly describe the details of an immediate allergic response, even after many years, especially if the symptoms were serious. If not, the claim of a 'food allergy' by a patient might be doubtful.
OFC testing should be considered if the patient's history is not convincing or the most recent allergic event occurred more than one year previously and the possibility of exhibiting tolerance is expected.
The general methodology of an OFC test is to administer the suspected food in gradually increasing doses under a medical setting [22]. An open challenge refers to an OFC test in which the patient can recognize the target food without blinding. The results can be definitive if the challenge yields either negative results or positive results with objective symptoms. For most clinical settings, open challenges may be adequate because most patients are infants or young children in whom objective symptoms can be provoked. However, if the patient complains of subjective symptoms only, such as abdominal pain or pruritus, a blind challenge should be considered.
A double-blind placebo-controlled food challenge in which both the patient and the observer are blinded to the challenge material is the gold standard for diagnosing food allergies for both clinical and scientific purposes [23].
In the JPGFA 2012, the aims of OFC testing are categorized into three parts: diagnosis of the food allergy, determination of tolerance to the allergic food and a risk assessment.
Diagnostic OFC testing is typically performed after an elimination test in which the patient avoids the suspected food for several weeks to achieve relief of chronic eczema or gastrointestinal symptoms. After the symptoms have disappeared, an OFC test is performed to confirm recurrence of the symptoms associated with consumption of the target food [24]. However, more frequently, diagnostic OFC testing is performed before an introduction of a sensitized food for the first time in life.
The determination of tolerance (outgrow) is the most frequent indication for OFC testing. Most infants with egg [25], milk [26], wheat [27] or soybean allergies tend to outgrow these allergies during childhood. Information regarding accidental exposure to allergens helps to determine whether the patient is indicated for OFC testing. If the patient has experienced a severe reaction within one year, OFC testing is not recommended. Information regarding the daily consumption of foods containing small amounts of the allergen is also helpful for determining the indications and procedures of OFC testing.
Allergies to peanuts [28], tree nuts [29], buckwheat and shrimp, especially in older children or adults, are thought to continue throughout life. OFC testing for these foods may not be indicated unless loss of sensitization is confirmed by negative results in a skin prick or sIgE test.
Risk assessment challenges are performed only in specialized institutes for highly sensitized patients with a history of severe reactions. Small amounts of the target food is given to confirm the threshold level and severity of symptoms in order to provide caregivers information regarding how much attention should be paid to the allergy in daily life. Risk assessment challenges can also be the beginning of oral immunotherapy [30].
OFC tests should be performed in a setting that is fully equipped for emergency treatment. Well-trained doctors or nurses should keep in touch with the patient throughout the procedure, and the contribution of a dietitian helps a great deal [31]. The risks and benefits of OFC testing should be discussed with the patient and parents, and written informed consent must be obtained.
The setting may be in-hospital; however, an outpatient office or clinic may also be good for some patients in whom severe reactions are not predicted. A safe, clean and comfortable environment, hopefully free from contact with other patients with infectious diseases, where the patient can spend a long period of time is required.
The patient must be stable in his or her allergic condition, including exhibiting control of bronchial asthma and atopic eczema, and be free from any acute illnesses. Antihistamines and any other medications should be discontinued for >72 h before the OFC test, with the exception of inhaled corticosteroids and topical corticosteroid ointments applied to small skin lesions.
The challenge protocol is definitively important for the accuracy and safety of OFC testing. The total provocation dose should be adequate to provoke symptoms while being restricted to avoid any severe reactions [32]. For patients with a history of severe reactions, a step-wise procedure may be considered in which a small dose challenge is followed by a full-dose challenge.
Typical challenge foods and total doses are listed in Table 2. The starting dose should be 1 g (1 mL) or less of the challenge food [33]. The typical challenge scheme is to divide the total dose into three to six incremental doubling doses. For example, 1, 2, 4, 8 and 16 g of boiled egg whites or 1, 5, 10, 25, 50 and 100 mL of milk. Challenges using smaller doses (such as 0.1 g (mL) for the starting dose) should be considered in patients deemed to be at risk for severe reactions [34].
The doses are generally administered every 15 to 30 min over 1 to 2 h. A longer dosing interval may be applied in severe patients or in those who have experienced late-onset allergic reactions following intake of the suspected food. If signs of a suspicious reaction appear, the next dose should be postponed to observe the progress of symptoms or the same dose should be repeated to avoid overloading.
The patient may stay in the hospital for more than 2 h after the final dose or the provoked symptoms disappear. Upon discharge, the patient must be instructed to monitor the possibility of late-onset symptoms, even after undergoing a negative (passed) challenge.
Allergic reactions observed during OFC testing include cutaneous, mucosal, respiratory, gastrointestinal (GI), cardiovascular and neurological symptoms (Table 3). Parallel to the allergic reactions observed with accidental intake, cutaneous symptoms are the most frequently observed symptoms in 80% of positive challenges, followed by respiratory (35%) and GI (25%) symptoms (Fig. 5).
Respiratory symptoms are common and must be treated properly. Coughing can be divided into two categories: dry and staccato coughing estimated to be of laryngeal origin, and productive coughing associated with wheezing or asthma [35].
Oral symptoms can include oral allergy syndrome induced by local absorption of water soluble allergens, typically fruits or vegetables [36]. However, oral symptoms sometimes indicate the beginning of systemic anaphylaxis. Therefore, when the patient claims discomfort or itchiness in the mouth or small lip swelling or redness appears around the mouth, the next dose should be postponed to observe the course of the patient's symptoms.
Neurological symptoms may be a sign of systemic reactions, particularly when a small child is violently frightened and crying or suddenly turns quiet [37]. Overwhelming tiredness and sleepiness associated with GI symptoms are sometimes observed in older children.
Anaphylaxis is defined as a serious allergic reaction that is rapid in onset and may cause death [38]. Typically, it is associated with rapidly progressing multiple organ symptoms; however, isolated hypotension is also a symptom of anaphylaxis (Table 4) [39].
Skin and mucosal symptoms can be treated with antihistamines (oral or parenteral). Beta-agonist inhalation may be applied to treat mild respiratory symptoms, and oxygen should be administered if the patient's oxygen saturation falls below 95%. Intramuscular adrenaline (0.01 mg/kg) is the first-line treatment for anaphylaxis. The effects of adrenaline may be observed within 5 min. If the effects are insufficient or the symptoms reappear after 10 to 15 min, repeat administration of intramuscular adrenaline may be considered and additional treatments such as intravenous fluids, parenteral antihistamines or corticosteroids should be applied (Fig. 6)
Based on the total dose and symptoms provoked during OFC testing, the patient can be instructed regarding restrictions or re-introduction of the challenge food. Even after a negative challenge, the amount of food intake at home should not exceed that of the total dose by at least several times to confirm safety.
A positive challenge does not always suggest the need for complete elimination [40]. Patients may introduce small amounts of the target food within the appropriate safety range, at 1-10% of the threshold level, in general.
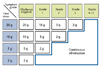
We usually perform OFC tests with boiled egg whites, milk and udon noodles for wheat using increasing doses of 1, 2, 5, 10 and 20-30 g every 20 min. Based on the final dose and the symptom grade, we determine the dose of initial intake (Fig. 7). If the patient is considered to be able to start with 2 g or more of the target food, he or she is instructed to take it at home. Continuous complete elimination is prescribed for patients who are not considered to be tolerant of 2 g of the target food.
In the follow-up visit conducted after one to two months, doctors and dietitians check the patient's food diary. If no adverse reactions are recorded, patients are instructed to increase the amount gradually. During repeated follow-up visits, typically conducted every two to three months, the patient is instructed to increase the dose of intake and to introduce the allergenic food into his or her daily meals at the safe levels.
The JPGFA 2012 emphasizes the principal policy for the management of food allergies as 'minimal allergen avoidance based on the appropriate diagnosis.' Evidence of the effects of oral immunotherapy supports the advantages of intake compared to the complete elimination of the allergenic food as far as the safety of the patient is concerned.
To achieve this goal, the purpose of 'diagnosing' a food allergy is not only to confirm whether the patient is allergic, but also to determine the 'safety level' of consumption. At this time, OFC testing is the only diagnostic procedure that can determine the threshold level and severity of symptoms. Even after a positive challenge, providing quantitative diet instructions enables the patient to introduce the allergenic food. Repeated evaluations and diet instructions may achieve release from an elimination diet much sooner than simply maintaining the elimination diet.
Figures and Tables
Fig. 1
Probability curves of egg white- and milk-specific IgE antibodies. Age-related probability curves of allergen-specific IgE reactions to egg whites (A) and milk (B) for patients failing oral food challenge testing for heated eggs and milk, respectively. Adapted from reference [13].

Fig. 2
Probability curve for the initial diagnosis of egg allergies. Probability curves of allergen-specific IgE reactions to egg whites (n = 100, linear line) and ovomucoid (n = 80, dotted line) for patients failing boiled egg challenge testing based on testing performed in 1-year-old patients who had never eaten egg products. Adapted from reference [14].

Fig. 3
Receiver operating characteristic (ROC) curves showing the results of allergen-specific IgE (sIgE) tests for milk components in relation to the diagnosis of milk allergies. Milk-sensitized children were diagnosed with a cow's milk allergy (CMA, n = 61) or non-CMA (n = 22). ROC analyses were performed for the sIgE tests to milk, casein, α-lactalbumin, and β-lactoglobulin. Adapted from reference [15].
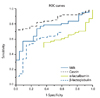
Fig. 4
Probability curves for wheat and ω-5 gliadin IgE. Probability curves of allergen-specific IgE reactions to wheat (A) and ω-5 gliadin (B) for the diagnosis of wheat allergies (n = 59) or clinically evaluated non-wheat allergies (n = 174). Adapted from reference [19].

Fig. 5
Provoked symptoms in positive food challenges. A total of 1,834 oral food challenge tests were performed at Aichi Children's Health and Medical Center between January 2006 and March 2009. The symptoms observed in the positive challenges (n = 717) are shown. In the respiratory symptoms, the blue bar indicates coughing, and the green bar indicates wheezing.
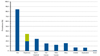
Fig. 6
Treatment plan for allergic symptoms. A flow chart of the treatment plan for patients with positive oral food challenge results is shown in the Japanese Pediatric Guideline for Food Allergy 2012.
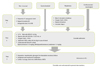
Fig. 7
Selection of the introduction dose after oral food challenge testing. According to the final dose of the challenge food (boiled egg whites, udon noodles or milk) and the symptom grading (Table 3), the initial dose of intake at home is determined.
ACKNOWLEDGEMENTS
The basic concept of this review was presented at the 62nd Korean Pediatric Society meeting (October 2012). This review was partially supported by a grant from the Ministry of Health, Labour and Welfare, 2012.
References
1. Ebisawa M, Sugizaki C. Prevalence of pediatric allergic diseases in the first 5 years of life. J Allergy Clin Immunol. 2008. 121:S237.

2. Umemura H, Kando N, Izumi H, Katou M, Ito K. Evaluation of school lunches of the pediatric patients with food allergies. Jpn J Pediatr Allergy Clin Immunol. 2012. 26:589–598.

3. McIntyre CL, Sheetz AH, Carroll CR, Young MC. Administration of epinephrine for life-threatening allergic reactions in school settings. Pediatrics. 2005. 116:1134–1140.

4. Borres MP, Ebisawa M, Eigenmann PA. Use of allergen components begins a new era in pediatric allergology. Pediatr Allergy Immunol. 2011. 22:454–461.

5. Berni Canani R, Ruotolo S, Discepolo V, Troncone R. The diagnosis of food allergy in children. Curr Opin Pediatr. 2008. 20:584–589.

6. García C, El-Qutob D, Martorell A, Febrer I, Rodríguez M, Cerdá JC, Félix R. Sensitization in early age to food allergens in children with atopic dermatitis. Allergol Immunopathol (Madr). 2007. 35:15–20.

7. Japanese Society of Pediatric Allergy and Clinical Immunology. Japanese Pediatric Guideline for Oral Food Challenge Test in Food Allergy 2009. 2009. Tokyo: Kyowa-Kikaku.
8. Ito K, Urisu A. Diagnosis of food allergy based on oral food challenge test. Allergol Int. 2009. 58:467–474.

9. Japanese Society of Pediatric Allergy and Clinical Immunology. Japanese Pediatric Guideline for Food Allergy 2012. 2011. Tokyo: Kyowa-Kikaku.
10. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001. 107:891–896.

11. Urisu A, Ando H, Morita Y, Wada E, Yasaki T, Yamada K, Komada K, Torii S, Goto M, Wakamatsu T. Allergenic activity of heated and ovomucoid-depleted egg white. J Allergy Clin Immunol. 1997. 100:171–176.

12. Ando H, Movérare R, Kondo Y, Tsuge I, Tanaka A, Borres MP, Urisu A. Utility of ovomucoid-specific IgE concentrations in predicting symptomatic egg allergy. J Allergy Clin Immunol. 2008. 122:583–588.

13. Komata T, Söderström L, Borres MP, Tachimoto H, Ebisawa M. The predictive relationship of food-specific serum IgE concentrations to challenge outcomes for egg and milk varies by patient age. J Allergy Clin Immunol. 2007. 119:1272–1274.

14. Haneda Y, Kando N, Yasui M, Kobayashi T, Maeda T, Hino A, Hasegawa S, Ichiyama T, Ito K. Ovomucoids IgE is a better marker than egg white-specific IgE to diagnose boiled egg allergy. J Allergy Clin Immunol. 2012. 129:1681–1682.

15. Ito K, Futamura M, Movérare R, Tanaka A, Kawabe T, Sakamoto T, Borres MP. The usefulness of casein-specific IgE and IgG4 antibodies in cow's milk allergic children. Clin Mol Allergy. 2012. 10:1.

16. Urisu A, Yamada K, Masuda S, Komada H, Wada E, Kondo Y, Horiba F, Tsuruta M, Yasaki T, Yamada M, Torii S, Nakamura R. 16-kilodalton rice protein is one of the major allergens in rice grain extract and responsible for cross-allergenicity between cereal grains in the Poaceae family. Int Arch Allergy Appl Immunol. 1991. 96:244–252.

17. Ito K, Futamura M, Borres MP, Takaoka Y, Dahlstrom J, Sakamoto T, Tanaka A, Kohno K, Matsuo H, Morita E. IgE antibodies to omega-5 gliadin associate with immediate symptoms on oral wheat challenge in Japanese children. Allergy. 2008. 63:1536–1542.
18. Morita E, Matsuo H, Mihara S, Morimoto K, Savage AW, Tatham AS. Fast omega-gliadin is a major allergen in wheat-dependent exercise-induced anaphylaxis. J Dermatol Sci. 2003. 33:99–104.
19. Otsuji K, Futamura M, Kando N, Hayashi K, Ito K. Clinical evaluation of ω-5 gliadin-specific IgE test. Arerugi. 2011. 60:971–982.
20. Ito K, Morishita M, Ohshima M, Sakamoto T, Tanaka A. Cross-reactive carbohydrate determinant contributes to the false positive IgE antibody to peanut. Allergol Int. 2005. 54:387–392.

21. Ebisawa M, Movérare R, Sato S, Maruyama N, Borres MP, Komata T. Measurement of Ara h 1-, 2-, and 3-specific IgE antibodies is useful in diagnosis of peanut allergy in Japanese children. Pediatr Allergy Immunol. 2012. 23:573–581.

22. Nowak-Wegrzyn A, Assa'ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS. Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009. 123:S365–S383.
23. Rancé F, Deschildre A, Villard-Truc F, Gomez SA, Paty E, Santos C, Couderc L, Fauquert JL, De Blic J, Bidat E, Dupont C, Eigenmann P, Lack G, Scheinmann P. Oral food challenge in children: an expert review. Eur Ann Allergy Clin Immunol. 2009. 41:35–49.
24. Niggemann B. Role of oral food challenges in the diagnostic work-up of food allergy in atopic eczema dermatitis syndrome. Allergy. 2004. 59:Suppl 78. 32–34.

25. Savage JH, Matsui EC, Skripak JM, Wood RA. The natural history of egg allergy. J Allergy Clin Immunol. 2007. 120:1413–1417.

26. Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow's milk allergy. J Allergy Clin Immunol. 2007. 120:1172–1177.

27. Keet CA, Matsui EC, Dhillon G, Lenehan P, Paterakis M, Wood RA. The natural history of wheat allergy. Ann Allergy Asthma Immunol. 2009. 102:410–415.

28. Savage JH, Limb SL, Brereton NH, Wood RA. The natural history of peanut allergy: Extending our knowledge beyond childhood. J Allergy Clin Immunol. 2007. 120:717–719.

29. Fleischer DM, Conover-Walker MK, Matsui EC, Wood RA. The natural history of tree nut allergy. J Allergy Clin Immunol. 2005. 116:1087–1093.

31. Wood RA. Adkinson NF, Busse WW, Bochner BS, Holgate ST, Simons FE, Lemanske RF, editors. Oral food challenge testing. Middleton's allergy: principles & practice. 2009. Philadelphia: Mosby;1309–1317.
32. Perry TT, Matsui EC, Conover-Walker MK, Wood RA. Risk of oral food challenges. J Allergy Clin Immunol. 2004. 114:1164–1168.

33. Taylor SL, Hefle SL, Bindslev-Jensen C, Atkins FM, Andre C, Bruijnzeel-Koomen C, Burks AW, Bush RK, Ebisawa M, Eigenmann PA, Host A, Hourihane JO, Isolauri E, Hill DJ, Knulst A, Lack G, Sampson HA, Moneret-Vautrin DA, Rance F, Vadas PA, Yunginger JW, Zeiger RS, Salminen JW, Madsen C, Abbott P. A consensus protocol for the determination of the threshold doses for allergenic foods: how much is too much? Clin Exp Allergy. 2004. 34:689–695.

34. Devenney I, Norrman G, Oldaeus G, Strömberg L, Fälth-Magnusson K. A new model for low-dose food challenge in children with allergy to milk or egg. Acta Paediatr. 2006. 95:1133–1139.

37. De Swert LF, Bullens D, Raes M, Dermaux AM. Anaphylaxis in referred pediatric patients: demographic and clinical features, triggers, and therapeutic approach. Eur J Pediatr. 2008. 167:1251–1261.

38. Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, Bahna SL, Beck LA, Byrd-Bredbenner C, Camargo CA Jr, Eichenfield L, Furuta GT, Hanifin JM, Jones C, Kraft M, Levy BD, Lieberman P, Luccioli S, McCall KM, Schneider LC, Simon RA, Simons FE, Teach SJ, Yawn BP, Schwaninger JM. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010. 126:S1–S58.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download