Abstract
Heparin has been widely used for intradialytic anticoagulation since the 1940s. Heparin induced anaphylaxis can be life threatening, mandating early recognition and intervention. However, due to its relative rarity many physicians remain unaware. We report the case of a 70-year-old woman requiring dialysis, who developed recurrent anaphylaxis to intradialytic heparin. We describe a systematic approach to confirm the suspected heparin allergy, which must include an evaluation of predisposing factors, the dialysis equipment and concomitant medications. Further workup for safe alternatives employing skin prick and intradermal tests, as well as provocation tests are discussed.
Unfractionated heparin has been widely used as an anticoagulant in end stage renal failure patients requiring hemodialysis since the 1940s [1]. While rare, heparin induced anaphylaxis is a potentially life threatening situation that mandates early recognition and intervention. However, due to its relative rarity [2] many physicians remain unaware. We present such a case, with its subsequent workup and rationale for further management.
A 70-year-old Chinese female with hypertensive kidney disease was deemed to require dialysis. She had no known allergies and never received angiotensin converting enzyme (ACE) inhibitors. Dialysis was commenced through a permanent catheter with 500 U of unfractionated heparin/hr for intradialytic anticoagulation. She tolerated 3 hemodialysis sessions per-week in the initial 2 weeks. The following week she developed hypotension and dyspnea at the start of a dialysis session. First use syndrome was the postulated cause and she was discharged after observation and treatment with intravenous hydrocortisone and antihistamines. However, she mounted a more severe reaction within minutes of starting the subsequent dialysis session two days later, with flushing, hypotension and rhonchi, requiring admission to the high dependency unit. A serum tryptase level by fluorescent enzyme immunoassay done immediately after the reaction was elevated at 43.1 µg/L (ref <11.4 µg/L). Heparin was thought to be the common inciting agent and the cause for her recurrent anaphylaxis. Furthermore, although no subsequent dialysis sessions with heparin were carried out, she developed urticaria on the following day. As heparin was used in the central catheter lock solution, it was postulated that systemic extension of heparin from within the tubing was responsible for this particular reaction. She was subsequently able to tolerate heparin-free dialysis, lending support to our hypothesis that heparin was indeed the culprit. In addition, citrate substituted heparin as catheter lock solution, and no further reactions were observed. Other variables, including the dialysis membrane and sterilant, were not modified. Further evaluation to confirm the suspected heparin allergy and determine safe alternatives for subsequent hemodialysis was indicated.
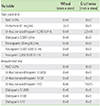
Skin prick tests were done with unfractionated heparin (5,000 U/mL), and its possible alternatives: dalteparin (2,500 U/mL), enoxaparin (20 mg/0.2 mL), tinzaparin (3,500 IU/0.35 mL) and fraxiparin (2,850 IU/0.35 mL) (Table 1). Histamine (0.1 mg/mL) and 0.9% normal saline solution were used as positive and negative controls. The prick test was positive for heparin but negative for the rest. This was further substantiated by a positive intradermal test with heparin at 1:10 dilution of the above concentration (negative at 1:1,000 and 1:100). Intradermal tests were negative for dalteparin at similar dilutions (1:1,000, 1:100 and 1:10). This was followed by subcutaneous and intravenous challenges with dalteparin at incremental concentrations as described in Table 2, reaching up to 1,250 U of intravenous dalteparin. She has tolerated hemodialysis with dalteparin for the past 2 years.
The workup of a patient with intradialytic anaphylactic reactions must include a systematic evaluation of possible causes [3]. One obvious cause of anaphylaxis is the dialysis membrane itself. First use syndrome is an anaphylactic reaction to the artificial kidney (either to the residual sterilant or material in the dialysis membrane), which is rare nowadays owing to increased standards of sterilization and use of membranes with higher biocompatibility [4]. Patients on ACE inhibitors are at higher risk of developing anaphylactoid reactions to the dialysis membrane due to the patients' inability to degrade bradykinin, which is produced on contact with the negatively charged dialysis membrane [5, 6]. A range of anaphylactic reactions had also been reported to heparin contaminated with oversulfated chondroitin sulfate in 2008, resulting in 80 deaths. Stringent manufacturing standards for heparin are now in place to ensure its quality [7]. Miscellaneous agents, such as iron or erythropoietin, which are frequently used in the management of anemia in chronic renal disease; as well as antiseptic preparations, including chlorhexidine and iodine, are additional factors worth considering [3].
Finally, as in our case, intradialytic anticoagulants must always be assumed to be a potential culprit. Systematic workup to confirm the causal agent is crucial to determine plans for subsequent dialysis sessions. Of note, there is cross-reactivity between the various heparins by virtue of their similar molecular structure [8], and workup should therefore include a thorough assessment for safe alternatives. Other groups have previously suggested directing work up for alternatives at anticoagulants outside of the main heparin family instead, such as the heparinoids [9]. Hirudins and fondaparinux [10] have also been tested as alternatives for general anticoagulation, but these may not be applicable in the setting of dialysis. Our focus was to work up for safe alternatives within the different types of heparin.
Delayed reactions to subcutaneously injected heparins are commonly seen and workup includes the use of patch and intradermal tests [10]. In contrast, immediate hypersensitivity reactions to systemically administered heparins are rare. We used skin prick tests to confirm the diagnosis of heparin allergy and identify potential alternative agents. Since dalteparin gave a negative reading on skin prick test, it was subjected to further testing via intradermal as well as subsequent subcutaneous and intravenous provocation tests. As presented in Table 1, the reaction incited by unfractionated heparin in the skin prick test was a flare, rather than the typical wheal expected in a positive SPT: skin prick test. However, allergy testing needs to be interpreted in the context of a given clinical scenario. In our patient, where the patient tolerated dialysis in the absence of heparin-while other variables remained constant-the flare reaction should be interpreted to have clinical significance.
There is debate on nonirritating concentrations to use for intradermal tests with the different heparins, though an intradermal test at 1:10 dilution has been suggested to be useful to identify true reactions while maintaining the sensitivity of the skin test [11-14]. To further increase the sensitivity and specificity of the skin tests, a thorough workup should include a range of low to high concentrations. We began with dilutions as low as 1:1,000 and slowly escalated to 1:10, increasing the safety and specificity of the protocol by doing skin prick tests before going on to do intradermal tests. We should remain cognizant of the potential for false positive reactions at higher concentrations. In our case, a 1:10 dilution incited a wheal with unfractionated heparin, but not with dalteparin, arguing against an irritant effect from the 1:10 concentration.
To our knowledge, this is the first report describing a systematic workup of the different types of heparin in a setting of recurrent intradialytic anaphylaxis to unfractionated heparin, with an aim to establish safe alternatives for subsequent dialysis. In our patient, removal of heparin prevented intradialysis anaphylaxis. If this intervention were not helpful, however, then a change in dialysis membrane would have been the appropriate next step.
As mentioned, cross reactivity between the different types of heparins have been well reported [8, 9]. Therefore, all potential alternatives need to be subjected to rigorous workup through skin and provocation tests to determine viable options for each patient. Occasionally, there is limited or no availability of alternative anticoagulants. For these situations, several groups have reported successful desensitization protocols with heparin, including in the dialysis setting [12, 15].
In conclusion, the workup of anaphylaxis in a dialysis setting warrants a systematic approach, including skin and provocation tests to the suspected agents and its alternatives.
Figures and Tables
References
1. Fellner SK, Purkerson ML. Gordon Murray: heparin, hemodialysis and hubris. Am J Nephrol. 2002; 22:271–277.

2. Ueda A, Nagase S, Morito N, Yotsumoto M, Ohba S, Hasegawa Y, Narita M, Koyama A. Anaphylactoid reaction induced by low-molecular-weight heparin in a hemodialysis patient. Nephron. 2001; 87:93–94.

3. Ebo DG, Bosmans JL, Couttenye MM, Stevens WJ. Haemodialysis-associated anaphylactic and anaphylactoid reactions. Allergy. 2006; 61:211–220.

4. Klinkmann H, Grassmann A, Vienken J. Dilemma of membrane biocompatibility and reuse. Artif Organs. 1996; 20:426–432.

5. Coppo R, Amore A, Cirina P, Scelfo B, Giacchino F, Comune L, Atti M, Renaux JL. Bradykinin and nitric oxide generation by dialysis membranes can be blunted by alkaline rinsing solutions. Kidney Int. 2000; 58:881–888.

6. Verresen L, Fink E, Lemke HD, Vanrenterghem Y. Bradykinin is a mediator of anaphylactoid reactions during hemodialysis with AN69 membranes. Kidney Int. 1994; 45:1497–1503.

7. Krauskopf L. FDA issues plan to avoid heparin contamination [Internet]. New York: Thomson Reuters;updated 2012 Feb 10. cited 2013 Mar 3. Available from: http://www.reuters.com/article/2012/02/10/us-fda-heparin-idUSTRE8190RB20120210.
8. Jappe U, Gollnick H. Allergy to heparin, heparinoids, and recombinant hirudin. Diagnostic and therapeutic alternatives. Hautarzt. 1999; 50:406–411.
9. Berkun Y, Haviv YS, Schwartz LB, Shalit M. Heparin-induced recurrent anaphylaxis. Clin Exp Allergy. 2004; 34:1916–1918.

10. Koch P. Delayed-type hypersensitivity skin reactions due to heparins and heparinoids. Tolerance of recombinant hirudins and of the new synthetic anticoagulant fondaparinux. Contact Dermatitis. 2003; 49:276–280.

11. Trautmann A, Seitz CS. The complex clinical picture of side effects to anticoagulation. Med Clin North Am. 2010; 94:821–834.

12. Kavut AB, Koca E. Successful desensitization with un-fractionated heparin in a patient with heparin allergy and tolerance to fondaparinux. Asian Pac J Allergy Immunol. 2012; 30:162–166.
13. Brockow K, Garvey LH, Aberer W, Atanaskovic-Markovic M, Barbaud A, Bilo MB, Bircher A, Blanca M, Bonadonna B, Campi P, Castro E, Cernadas JR, Chiriac AM, Demoly P, Grosber M, Gooi J, Lombardo C, Mertes PM, Mosbech H, Nasser S, Pagani M, Ring J, Romano A, Scherer K, Schnyder B, Testi S, Torres M, Trautmann A, Terreehorst I. ENDA/EAACI Drug Allergy Interest Group. Skin test concentrations for systemically administered drugs an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2013; 68:702–712.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download