Abstract
Fixed drug eruption is an uncommon adverse drug reaction caused by delayed cell-mediated hypersensitivity. Levocetirizine is an active (R)-enatiomer of cetirizine and there have been a few reports of fixed drug eruption related to these antihistamines. We experienced a case of levocetirizine-induced fixed drug eruption and cross-reaction with other piperazine derivatives confirmed by patch test. A 73-year-old female patient presented with recurrent generalized itching, cutaneous bullae formation, rash and multiple pigmentation at fixed sites after taking drugs for common cold. She took bepotastine besilate (Talion®) and levocetirizine (Xyzal®) as antihistamine. She took acetaminophen, pseudoephedrine 60 mg / triprolidine 2.5 mg (Actifed®), dihydrocodeinebitartrate 5 mg / di-methylephedrine hydrochloride 17.5 mg / chlorpheniramine maleate 1.5 mg / guaifenesin 50 mg (Codening®) and aluminium hydroxide 200 mg / magnesium carbonate 120 mg (Antad®) at the same time. Patch test was done with suspected drugs and the result was positive with levocetirizine. We additionally performed patch test for other antihistamines such as cetirizine, hydroxyzine, fexofenadine and loratadine. Piperazine derivatives (cetirizine and hydroxyzine) were positive, but piperidine derivatives (fexofenadine and loratadine) were negative to patch test. There was no adverse drug reaction when she was challenged with fexofenadine. We report a case of levocetirizine-induced fixed drug eruption confirmed by patch test. Cross-reactions were only observed in the piperazine derivatives and piperidine antihistamine was tolerant to the patient.
H1-antihistamines act as antagonists to H1-receptor, and have antiallergic and antiinflammatory activities [1]. These agents have been classified into six chemical groups: the ethanolamines, ethylenediamines, alkylamines, piperazines, piperidines, and phenothiazines [1]. Most adverse effects of antihistamines are caused by their own binding activities to H1-receptors, muscarinic receptors, α-adrenergic receptors, serotonin receptors and cardiac ion currents [1]. These mechanisms may cause drowsiness, impairment of cognitive function, dry eyes, dry mouth and urinary retention [1]. Hypersensitivity to H1-antihistamine is rare, and there have been a few case reports of maculopapular eruption, fixed drug eruption and acute urticaria [2-7]. Here, we report a case of levocetirizine induced fixed drug eruption and cross-reactions with other antihistamines which have similar chemical structure.
A-73-year-old female patient visited our clinic with multiple round well-demarcated dark pigmented lesions with desquamation. She took medications because of common cold eighteen days ago. Medications were bepotastine besilate (Talion®; Mitsubishi Tanabe Pharma, Japan), levocetirizine (Xyzal®; UCB Korea Co., Ltd, Korea), acetaminophen, pseudoephedrine 60 mg / triprolidine 2.5 mg (Actifed®; Samil Pharm. Co., Ltd, Korea), dihydrocodeine bitartrate 5 mg / di-methylephedrine hydrochloride 17.5 mg / chlorpheniramine maleate 1.5 mg / guaifenesin 50 mg (Codening®; Chong Kun Dang Pharmaceutical Corp., Korea) and aluminium hydroxide 200 mg / magnesium carbonate 120 mg (Antad®; Hanbul Pharm Co., Ltd, Korea). After taking these medications, the patient experienced generalized itching and multiple erythematous macules with several bullae formation in about two h. These cutaneous lesions were spontaneously resolved after stopping taking medications and changed to pigmented lesion with desquamation. The patient had already experienced similar adverse reactions twice after taking bepotastine besilate, levocetirizine, acetaminophen, Actifed®, Codening®, Antad®, dexibuprofen and roxithromycin one and a half years ago. Multiple cutaneous erythema and bullae occurred and were resolved after two weeks with localized pigmentation.
The patient was a house wife and had diabetes mellitus and penicillin induced acute hypersensitivity. She denied alcohol intake and smoking.
In laboratory findings, complete blood cell counts were as follows; white blood cell 8,600/mm3 (neutrophil 76.6%, lymphocyte 15.7%, monocyte 8.8%, eosinophil 4.5%, basophil 0.6%), hemoglobin 11.9 g/dL, platelet 207,000/µL. C-reactive protein was 1.0 mg/dL. Hepatic enzymes, blood urea nitrogen and serum creatinine were within normal ranges. Patch test was done with suspected drugs such as bepotastine besilate, levocetirizine, acetaminophen, Codening®, codein, Actifed® and Antad® at both normal skin and pigmented skin. Petroleum (Vaseline®) was used to make appropriate concentration to test and control. All drugs were made to 10% concentration except codein 5% and additional Actifed® 1% concentration [8]. After 48 h, patch was removed and readings were performed 48 h after initial patch applying. At the normal skin site where levocetirizine had been applied, erythema was presented (Fig. 1A). At the pigmented skin site where levocetirizine had been applied, infiltration and vesicle were presented (Fig. 1B). We additionally performed patch test for other antihistamines including levocetirizine (5% and 10% of Pet.), cetirizine (10% of Pet.), hydroxyzine (10% of Pet.), ebastine (10% of Pet.), loratadine (10% of Pet.) and fexofenadine (10% of Pet.). Erythema was observed at patch test sites of cetirizine and hydroxyzine which were piperazine derivatives (Figs. 2A and B). But the patch tests of ebastine, fexofenadine and loratadine which were piperidine derivatives showed negative response (Figs. 2A and B). The open oral challenge test with fexofenadine was done. The patients took 120 mg fexofenadine a day for 3 days as the open oral challenge test, and there was no adverse reaction.
The patient was diagnosed as levocetirizine-induced fixed drug eruption which was confirmed by patch test. She has cross-reactions with other piperazine derivatives such as cetirizine and hydroxyzine. We recommended that the patients avoid taking these antihistamines. Fexofenadine could be an alternative drug without adverse reaction to patient.
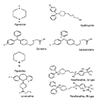
Levocetirizine is the single R-isomer of cetirizine dihydrochloride, and has twice of the affinity for the H1-receptor compared to cetirizine [9]. The chemical structures were shown in Fig. 3. Levocetirizine was noted as safe and effective treatment for allergic disease such as allergic rhinitis and chronic urticaria [9].
There was a few reports of piperazine antihistamine induced delayed hypersensitivity reaction [2-4, 6, 7]. One case-series was reported that hydroxyzine induced fixed drug eruption on the penis in four children [3]. Rechallenges were done and result in the same drug eruption [3]. In 2002, Assouère et al. [2] reported that the hydroxyzine induced the same morphologic cutaneous eruption at the same site which cetirizine had induced drug eruption before. Interestingly, two drugs are piperazine antihistamines. In 2007, Mariana et al reported one case of fixed drug eruption to cetirizine [4]. The results of patch test with cetirizine, levocetirizine and hydroxyzine which were all piperazine antihistamines were positive. These results could be evidences that delayed type antihistamine induced hypersensitivity showed cross-reactions between similar chemical structures.
A few cases of immediate hypersensitivity reactions were also reported [6, 10, 11]. In 2006, González de Olano et al. [10] reported a cetirizine-induced acute urticaria which was confirmed by oral provocation test, although the skin prick test was negative. In 2009, a case of cetirizine induced anaphylaxis was reported [12]. Severe breathlessness, urticarial eruption, loss of consciousness and hypotension were developed within 15 min after oral ingestion of cetirizine, and recovered after epinephrine injection. That was the first exposure to the patient, and the mechanism of anaphylaxis induced by cetirizine was unclear [12].
In the first-generation antihistamines, hydroxyzine, buclizine, cyclizine and meclizine belong to piperazines [1]. In the second generation antihistamines, cetirizine and levocetirizine belong to piperazines [1]. Azatadine, cyproheptadine, diphennylpyraline and ketotifen belong to piperidines as the first-generation antihistamines, and astemizole, desloratadine, ebastine, fexofenadine, levocabastine, loratadine, mizolastine, olopatadine and terfenadine belong to piperidine as the second-generation antihistamines [1]. In the present case, the patient has a levocetirizine induced fixed drug eruption, and the piperazine derivatives such as cetirizine and hydroxyzine showed cross-reactions on the patch test. Interestingly, antihistamines which are piperidine derivatives such as ebastine, fexofenadine and loratadine did not show cross-reaction on the patch test. To confirm the safety of alternative candidate drug, oral challenge was performed with fexofenadine. The patient was tolerable even after taking 120 mg fexofenadine for 3 days, and there was no additional adverse reaction.
Fixed drug eruption usually appears as a small number of pruritic, well circumscribed, erythematous macules [13]. These lesions typically recur at the same site and resolved spontaneously after discontinuation of causative drug [13]. Fixed drug eruption is considered as a form of classic delayed-type hypersensitivity mediated by CD8+ T cells [13]. In a previous report, during the initial phase of fixed drug eruption reactions, mast cells are thought to contribute to the activation of intraepidermal CD8+ T cells through the induction of cell adhesion molecules on keratinocytes [13]. The similar chemical structure may be recognized by T cell receptor or mast cell receptor.
Oral challenge test and patch test are usually performed to diagnose fixed drug eruption [13]. The results are graded from negative reaction to extreme positive reaction with intense erythema and coalescing vesicles [14]. Patch test should be done at the site of previous lesion and need a sufficient time to avoid refractory period [13, 15]. These considerations could decrease false negative results. The lymphocyte transformation test (LTT) is also reliable to identify the causative drug in many types of delayed drug eruptions [16]. But, the LTT was not done in this study, since positive LTT reactions are rarely obtained in patient with fixed drug eruption [13]. Oral challenge test is the most reliable method for diagnosis, but we could diagnose the patient as levocetirizine induced fixed drug eruption based on the history of repeated characteristic adverse reactions after taking levocetirizine and the result of patch test.
In summary, we report a levocetirizine induced fixed drug eruption, showing cross-reaction with antihistamines sharing similar chemical structure in patch test. Antihistamines which have different chemical structures such as fexofenadine or lorantadine could be alternatives. Oral challenge test with fexofenadine was tolerable in our patient. In a patient who has hypersensitivity to a certain antihistamine, approaches to evaluate cross-reaction with other antihistamines and with safe drugs for alternative are needed.
Figures and Tables
Fig. 1
Patch test was done with talion (10% of Pet.), levocetirizine (10% of Pet.), acetaminophen (10% of Pet.), codening (10% of Pet.), codein (5% of Pet.), actifed (10% of Pet.) and antid (10% of Pet.) at both normal skin and pigmented skin. (A) At the site of levocetrizine applied on normal skin after 48 h, erythema was presented; (B) At the pigmented skin after 48 h, infiltration and vesicle were presented at the site of levocetirizine.
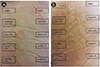
Fig. 2
Patch test for antihistamines including levocetirizine (5% and 10% of Pet.), cetirizine (Zyrtec®, 10% of Pet.), ebastine (Ebastel®, 10% of Pet.), loratadine (Clarityne®, 10% of Pet.), hydroxyzine (10% of Pet.), fexofenadine (10% of Pet.) and loratadine (10% of Pet.) were done. (A), (B) At the sites of cetirizine, levocetizine and hydroxyzine which were piperazine derivatives after 48 h, erythema was presented.

Fig. 3
Structures of anti-histamine; piperazine derivatives and piperidine derivatives. Images were extracted from website (http://en.wikipedia.org).
References
2. Assouère MN, Mazereeuw-Hautier J, Bonafé JL. [Cutaneous drug eruption with two antihistaminic drugs of a same chemical family: cetirizine and hydroxyzine]. Ann Dermatol Venereol. 2002; 129:1295–1298.
3. Cohen HA, Barzilai A, Matalon A, Harel L, Gross S. Fixed drug eruption of the penis due to hydroxyzine hydrochloride. Ann Pharmacother. 1997; 31:327–329.

4. Cravo M, Gonçalo M, Figueiredo A. Fixed drug eruption to cetirizine with positive lesional patch tests to the three piperazine derivatives. Int J Dermatol. 2007; 46:760–762.

5. Dwyer CM, Dick D. Fixed drug eruption caused by diphenhydramine. J Am Acad Dermatol. 1993; 29:496–497.

6. Kränke B, Mayr-Kanhäuser S. Urticarial reaction to the antihistamine levocetirizine dihydrochloride. Dermatology. 2005; 210:246–247.

7. Mahajan VK, Sharma NL, Sharma VC. Fixed drug eruption: a novel side-effect of levocetirizine. Int J Dermatol. 2005; 44:796–798.

8. Barbaud A. Skin testing in delayed reactions to drugs. Immunol Allergy Clin North Am. 2009; 29:517–535.

10. González de Olano D, Roán Roán J, de la Hoz Caballer B, Cuevas Agustín M, Hinojosa Macías M. Urticaria induced by antihistamines. J Investig Allergol Clin Immunol. 2006; 16:144–146.
11. Schröter S, Damveld B, Marsch WC. Urticarial intolerance reaction to cetirizine. Clin Exp Dermatol. 2002; 27:185–187.

12. Afonso N, Shetgaonkar P, Dang A, Rataboli PV. Cetirizine-induced anaphylaxis: a rare adverse drug reaction. Br J Clin Pharmacol. 2009; 67:577–578.

13. Shiohara T. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol. 2009; 9:316–321.

15. Barbaud A, Gonçalo M, Bruynzeel D, Bircher A. Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermatitis. 2001; 45:321–328.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download