Abstract
Immediate-type drug hypersensitivity is an increasingly significant clinical issue; however, the diagnosis is frequently hindered due to lack of safe and precise diagnostic tests. Flow cytometry-assisted basophil activation test is a safe in vitro diagnostic tool for assessing basophil activation upon allergen stimulation. In this review, we have summarized current literature on the diagnostic utilities, new indications, and methodological aspects of the basophil activation test for the diagnosis of drug hypersensitivity.
Go to : 
Flow cytometry-assisted basophil activation test (BAT) has been utilized in the diagnosis of immediate-type drug hypersensitivity from the early 1990s, when CD63 was discovered as a marker of basophil activation by Knol et al. [1]. This method has been further refined [2], owing to which the clinical applications of BAT have expanded [3].
However, immediate-type drug hypersensitivity is still a major diagnostic challenge to allergists and clinicians, e.g., penicillin allergy [4]. The challenge for diagnosis exists because there are insufficient methods to assess causal relationships. Drug provocation tests (DPTs) are the gold standards in hypersensitivity testing; however, they cannot always be administered due to the risks of systemic reactions [5]. Drug skin tests have recently been standardized and are reliable [6, 7]; however, except for a few well-known drugs, they have limited utility due to low sensitivity and specificity (e.g., skin irritations) [6]. In vitro allergen-specific IgE testing is another diagnostic option, but it may not be available for drugs other than beta-lactams.
In this review, we discuss the diagnostic potential of BAT in drug hypersensitivity. Although BAT is more expensive and technically challenging compared to conventional in vitro or in vivo tests, it can simultaneously and safely assess multiple drug responses. In addition, it directly measures basophil responses instead of immunoglobulin E (IgE) sensitization. Recent studies suggest that the applications of BAT can be extrapolated to additional drugs. The present review aims to summarize the current literature on the applications and methodological considerations of BAT in drug hypersensitivity.
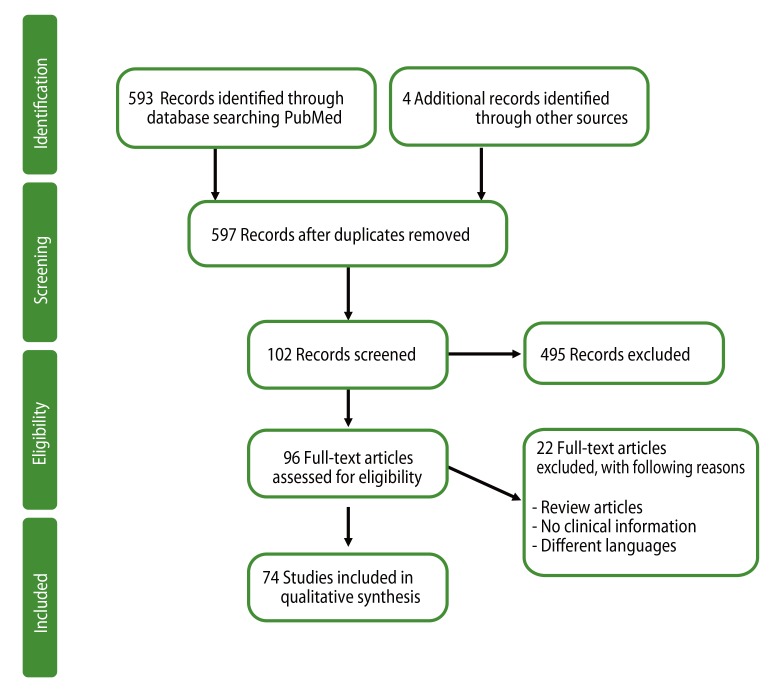
A systematic search strategy was adopted, in order to summarize the currently available literature. PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) searches were carried out using search terms basophil activation in titles and/or abstracts, for the period from January 1990 to August 2013. A manual search, using the same keywords, in Google Scholar (http://scholar.google.com/) was performed to identify additional papers. The search process followed the recommendations of the PRISMA statement (Fig. 1) [8], and was confined to articles with full-text accessibility. The present review includes analyses from 74 relevant papers, including original articles and case reports.
Go to : 
Beta-lactam antibiotics and neuromuscular blocking agents (NMBAs) were the first drugs for which BAT was applied. Aspirin and non-steroidal anti-inflammatory drugs (NSAIDs) are another class of drugs for which BAT was utilized. Recently, applications of BAT have extended to fluoroquinolones, radiocontrast media (RCM), and novel drugs such as anti-neoplastic or biologic agents.
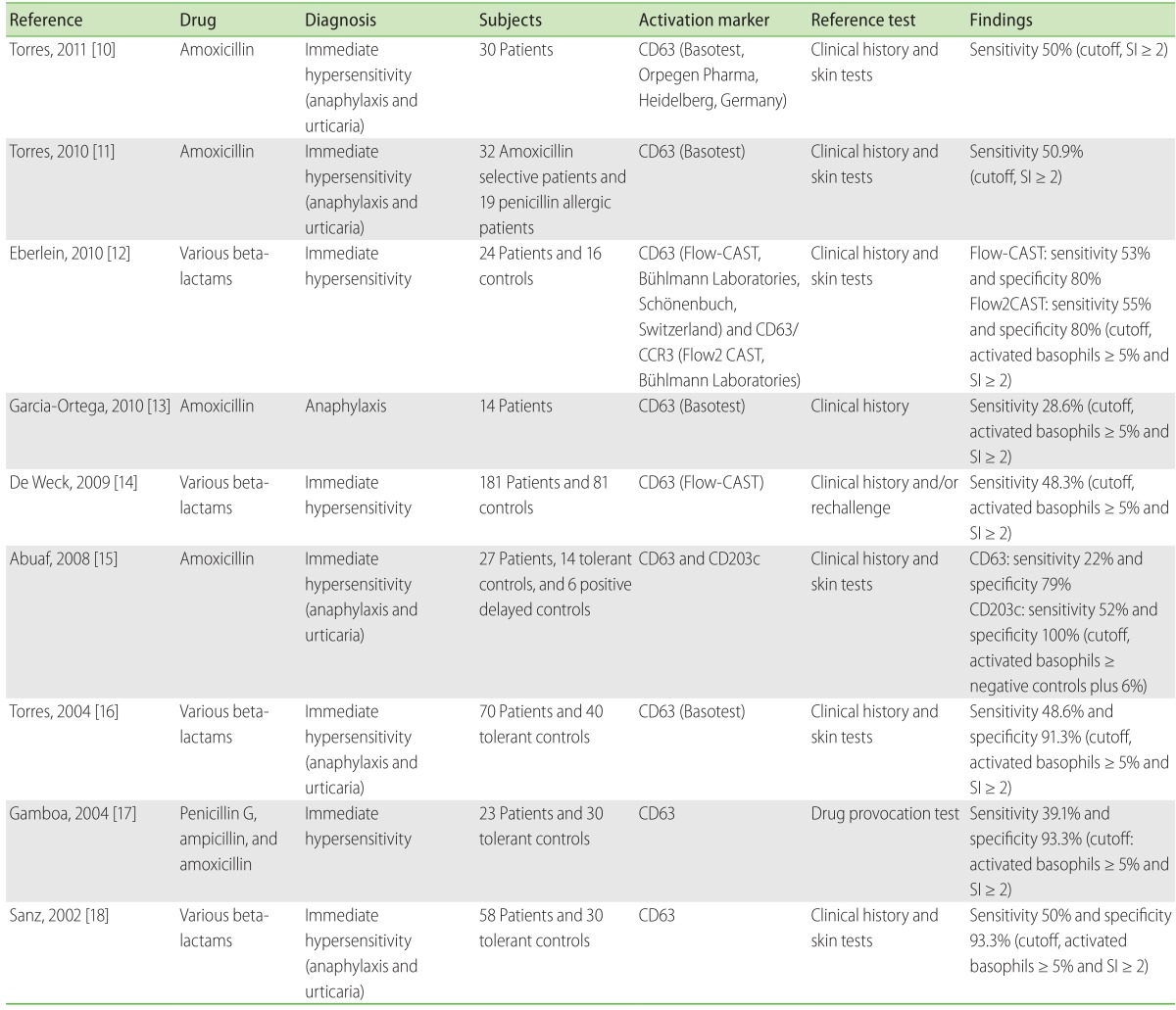
Conventionally, diagnoses of beta-lactam antibiotic hypersensitivities have been based on patient's clinical history and positive skin tests, or specific IgE antibody measurements [9]. To date, nine studies [10-18] have described the utility of BAT for diagnoses of beta-lactam allergies (Table 1). The sensitivities ranged from 28.6% to 55%; however, several large-scale studies have consistently demonstrated the sensitivity to be approximately 50%, in patients with positive clinical history and skin tests. Interestingly, the sensitivity of BAT was approximately 10% higher than that of the commercial specific IgE tests [14, 17, 18], and the specificity was more than 90%, clearly indicating that a positive BAT result was clinically significant. Importantly, BAT was positive in 25% of patients with positive provocation test and negative for specific IgE [17], and in 37% of patients with positive clinical history but negative skin tests [14]. These results suggest that BAT should be administered in cases where the diagnosis of drug allergy is highly suspected but is not supported by results of skin testing or in vitro IgE measurements. Because specific IgE tests are not available for most cephalosporins, BAT can be developed further for diagnosing allergies to a wider range of beta-lactams [9].
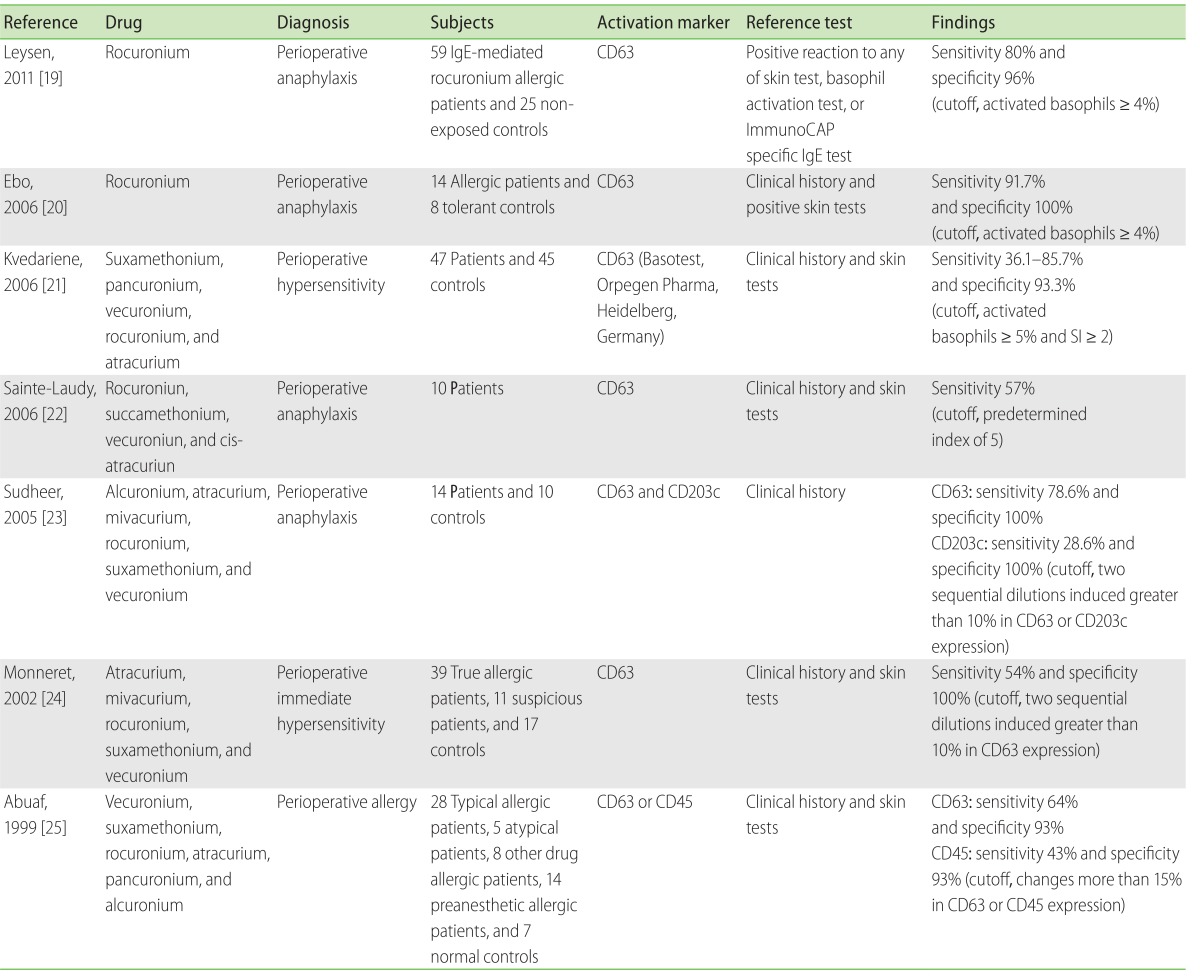
Currently, data for evaluating BAT results from patients with a history of perioperative hypersensitivity are available from seven clinical trials [19-25]. The sensitivity of BAT varied from 36.1% to 91.7% (Table 2); however, there was considerable heterogeneity in the inclusion criteria and cutoff levels. In patients with proven NMBA anaphylaxis, the BAT sensitivity was primarily 36.1%, which increased to 85.7% when allergies with an onset of less than 3 years were separately considered [21]. In the same patients, BAT showed high correlations with skin prick tests [20, 23, 26], better sensitivity [23], and higher specificity (range, 93% to 100%). Therefore, the time elapsed between the anaphylaxis and in vitro basophil activation [21] is a significant parameter for analyzing BAT sensitivity. In addition, BAT also plays an important complementary role in identifying cross-reactivity and safe alternatives in these patients [19-21, 23, 27].
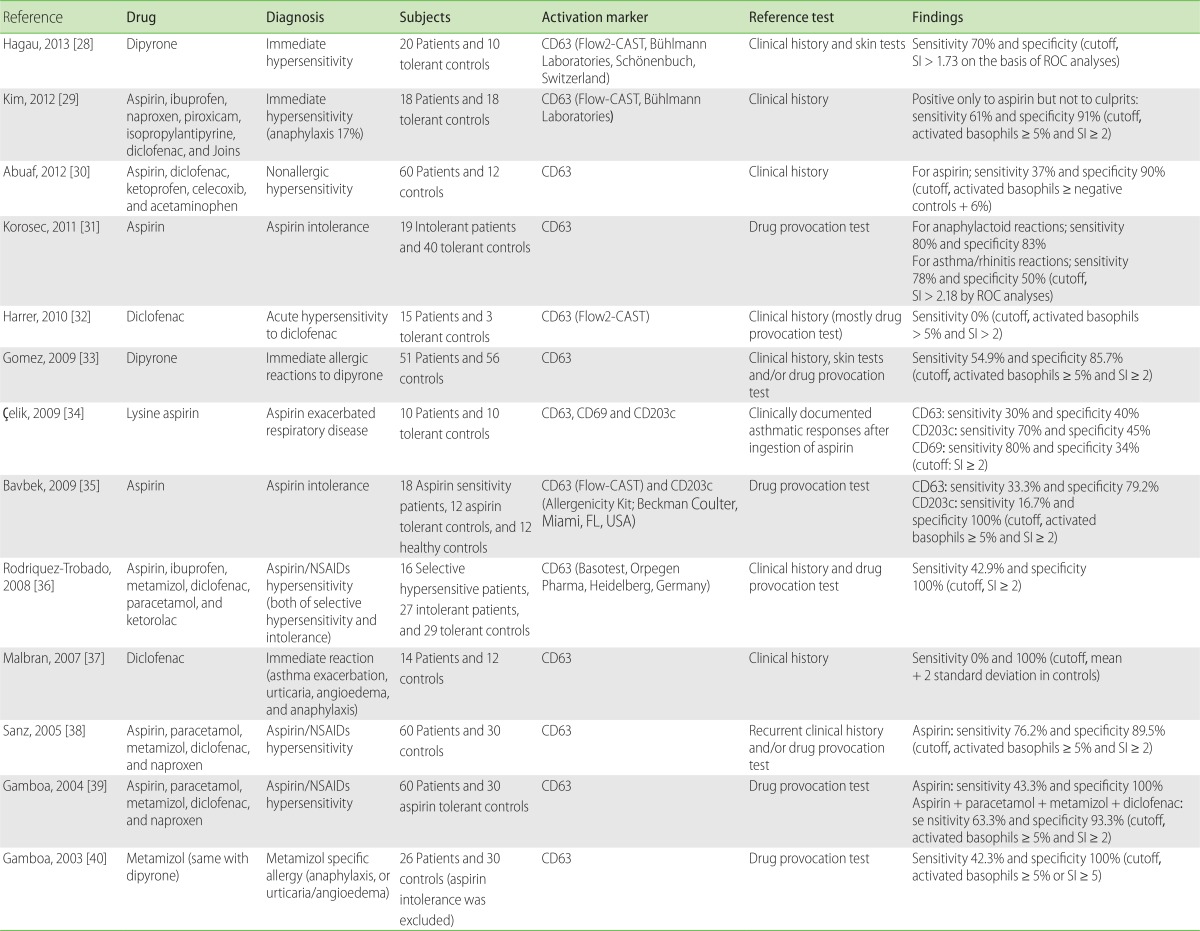
Aspirin or NSAIDs hypersensitivity is a heterogeneous disorder, encompassing IgE-mediated allergic reactions and non-immunological intolerances. The results with BAT on aspirin/NSAIDs hypersensitivity are conflicting or inconclusive (Table 3) [28-40]. Aspirin intolerance is mediated by the pharmacological effects on cyclooxygenase enzyme inhibition; therefore, it may not be a usual indication for BAT. It was discovered that BAT was not useful in patients with mild or cutaneous reactions, but it could only be indicated for severe reactions [30, 31]. In patients with aspirin intolerance, the combination of CD63 and CD203c measurements did not enhance the test sensitivity, which remained at 33.3% [35]. De Weck et al. [41] have questioned the proper interpretation on two earlier positive reports [38, 39]. Release of tryptase and histamine in response to oral challenges with aspirin suggested that circulating basophils play a role in aspirin intolerance [42]. However, these relationships are dose-dependent and likely to be mediated by the pharmacological inhibition of synthesis of prostaglandin E2, a natural inhibitor of basophil activation [41]. Therefore, BAT in aspirin intolerance may have to be sophisticated further to enhance the differences in dose responses between patients and controls. As diclofenac and naproxen have stronger in vitro pharmacological activity than aspirin, their inclusion has been suggested for enhancing the sensitivity of BAT [41].
Specific allergy to dipyrone has been evaluated by BAT [28, 33, 40]. Sensitivity and specificity ranged from 42.3% to 70% and 85.7% to 100%, respectively, depending on the cutoff values. A propyphenazone allergy case, which was diagnosed by BAT after human serum albumin (HSA) conjugation, has been previously reported [43]. However, in patients with selective diclofenac allergies, either diclofenac- or HSA-conjugated metabolites did not trigger CD63 expression [32].
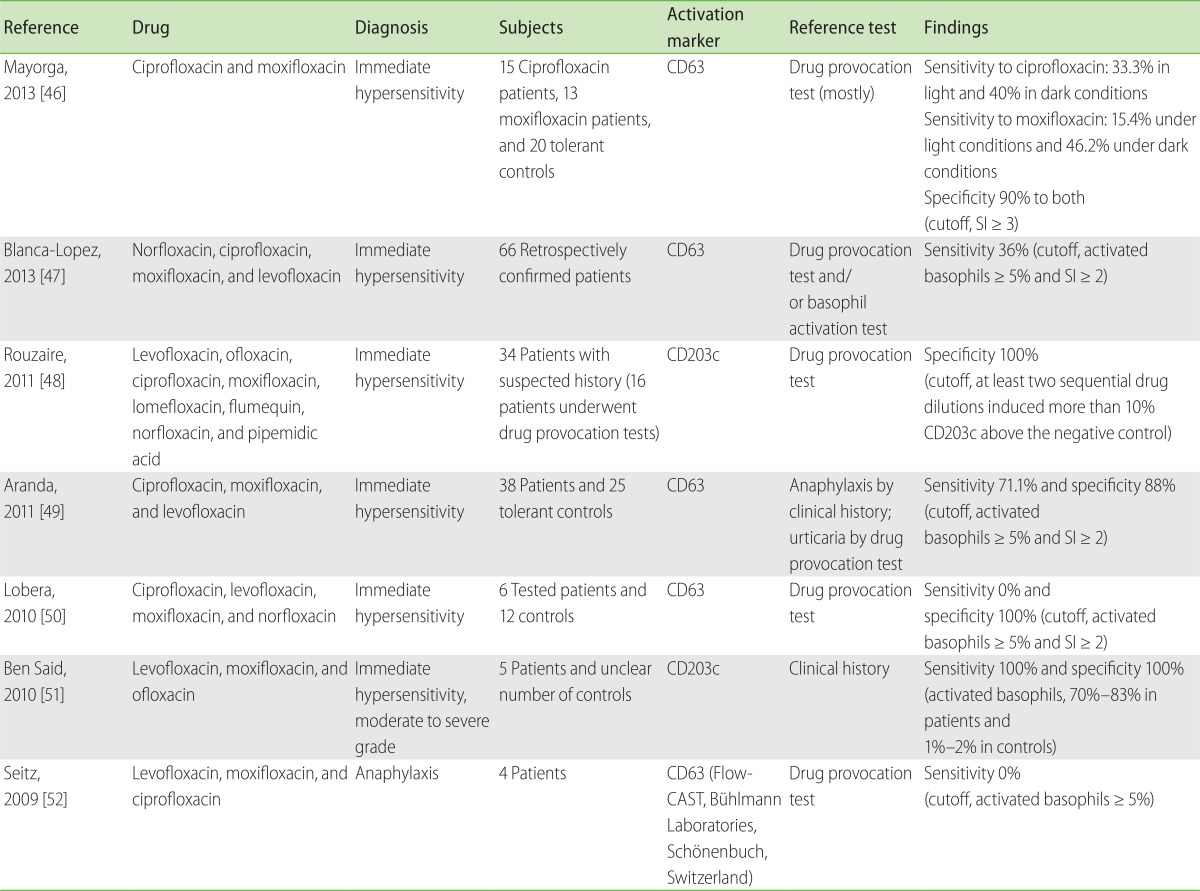
Fluoroquinolones, in addition to beta-lactams, cause one of the most common antibiotic allergies, and this hypersensitivity has become increasingly common with increased prescription rates of the drug [44]. BAT has gained considerable interest for testing fluoroquinolone hypersensitivities because the diagnostic utility of skin tests is very limited due to its skin-irritation properties in intradermal tests (88% false positives) [45]. To date, seven studies [46-52] have investigated the diagnostic utility of BAT (Table 4). The first study reported no positive BAT results in four DPT-proven patients [52]. Similarly, negative findings were reported in another study (n = 4, 0% positivity) [50]; however, larger scale studies performed later contradicted these findings. Another group discovered 70%-83% up-regulation of CD203c upon drug stimulation in all five participants with a history of anaphylaxis [51]. Other studies confirmed these findings by showing 71.1% sensitivity in 38 patients [49], and 36% sensitivity in 66 patients [47]. The excellent negative predictive value for DPT outcomes advocates the high utility of BAT in patients with suspected history of fluoroquinolone hypersensitivity [48].
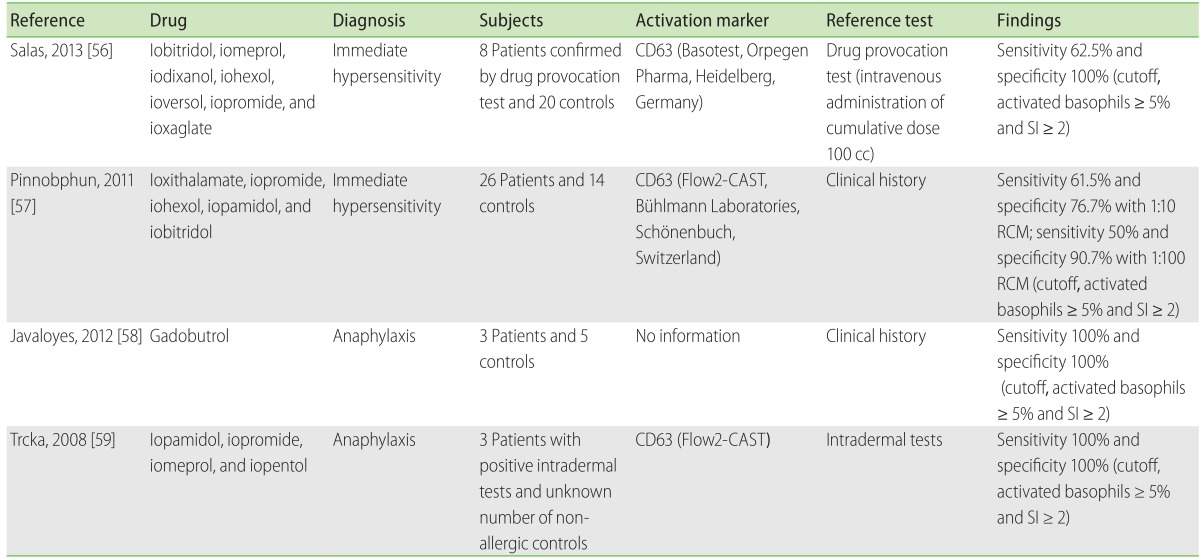
RCM hypersensitivity is a commonly encountered adverse drug reactions, and is the most common cause for anaphylaxis at a referral hospital in Korea [53]. Despite the introduction of non-ionic contrast media, the incidence of immediate hypersensitivity and severe reactions still appear as frequent as 2.1% and 0.01% per exposure, respectively [54]. Although skin testing is a relevant diagnostic method to determine the cause of hypersensitivity, it was meaningful only among patients with a history of moderate to severe hypersensitivity (40% positive in intradermal tests) [55]. Moreover, skin testing cannot detect non-IgE mediated RCM reactions.
Several studies [56-59] so far have analyzed the diagnostic value of RCM BAT (Table 5). Initial studies by Pinnobphun et al. [57] found the sensitivity to be 46.2%-61.5% and specificity 88.4%-100%, depending on the cutoff values. Recent studies reported the BAT sensitivity to be 62.5% compared to the outcome from intravenous challenges (n = 8), thereby confirming previous findings [56]. Interestingly, the skin test positivity did not correlate with BAT results, and BAT positivity did not correlate with the severity of reactions [57]. These findings suggest complementary roles for BAT in the diagnosis of RCM hypersensitivity. Further studies are necessary to understand its negative predictive values and to identify the precise mechanism for predicting safe alternative RCM in high-risk patients.
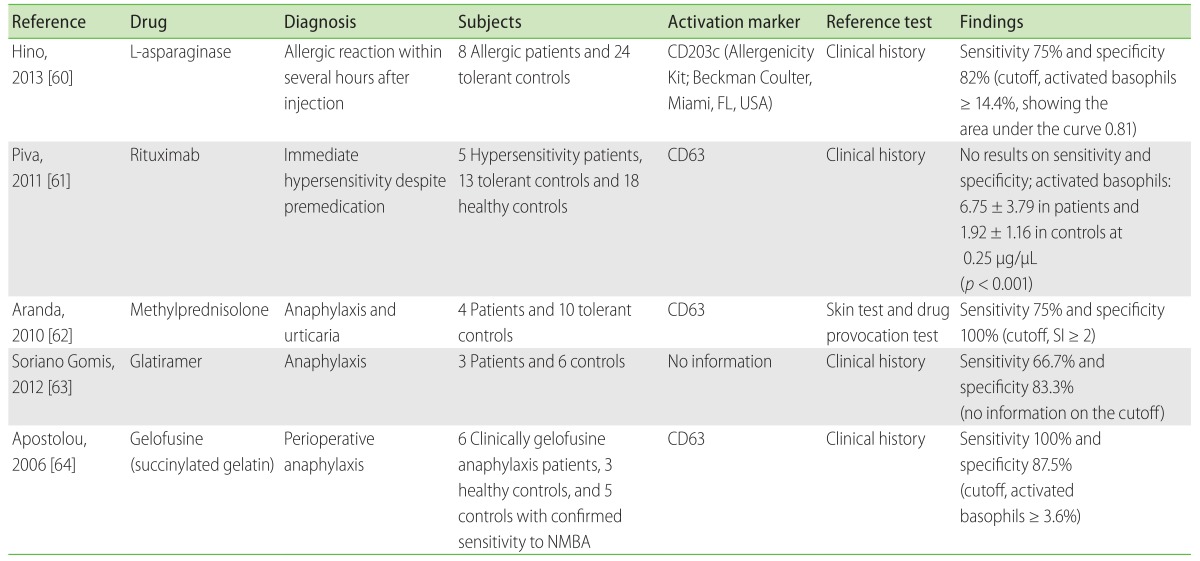
Recent studies [60-64] examined the outcome of BAT in patients with hypersensitivities to antineoplastic, biologic agents, or other drugs (Table 6). L-Asparaginase allergies were assessed using CD203c expression and were found to have high sensitivity (75%) and negative predictive value (96%) [60]. One case study also reported the potential utility of BAT in cisplatin hypersensitivity [65]. Because patients with malignancies may frequently have comorbidities or conditions that hamper skin testing, administering BAT will be advantageous in these cases.
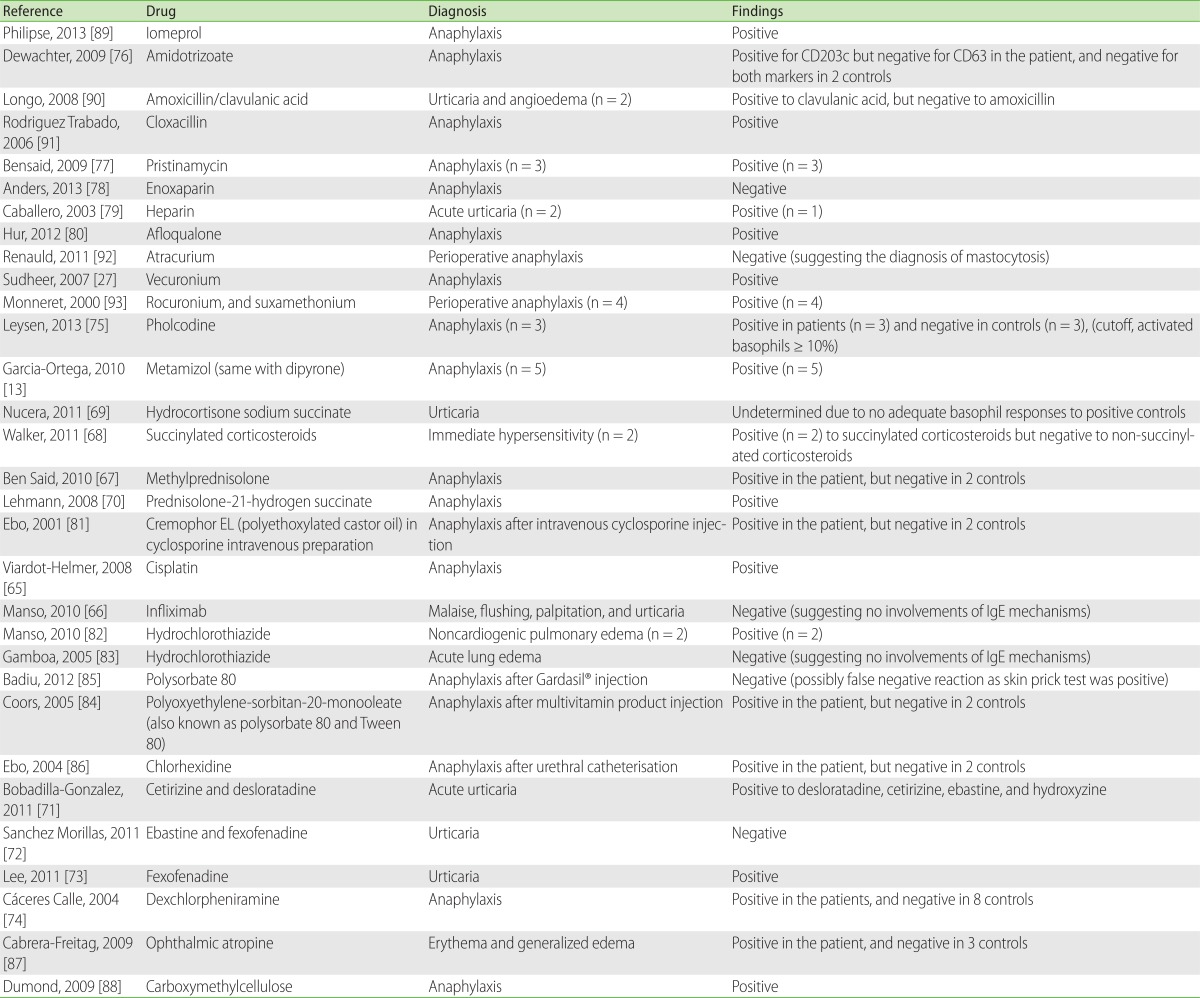
Hypersensitivity to other biologic agents such as rituximab [61] or infliximab [66] were examined by BAT, although the results warrant further confirmation. Among corticosteroids, methylprednisolone [62, 67] and succinylated corticosteroids [68-70] have been tested. Hypersensitivity to anti-histamines such as cetirizine, desloratadine, ebastine, fexofenadine, or dexchlorpheniramine was also assessed by BAT [71-74]. Other reports included testing for pholcodine [75], glatiramer [63], gelofusine [64], amidotrizoate [76], pristinamycin [77], enoxaparin [78], heparin [79], afloqualone [80], cremophor EL [81], hydrochlorothiazide [82, 83], polyoxyethylene (20) sorbitan monooleate [84, 85], chlorhexidine [86], ophthalmic atropine [87], and carboxymethylcellulose [88] in allergic or non-immunologic adverse reactions (Table 7) [13, 27, 61, 66-93]. Further studies are required for determining the causal relationships and identifying safe alternatives in patients with hypersensitivity to drugs that are not evaluated until date.
Go to : 
The theoretical and technical details of BAT have been extensively discussed before [2, 3, 41, 94-98]. Briefly, BAT is a flow cytometry-based cellular assay that measures the activation of basophils upon allergen stimulation. The activation response can be measured at a single-cell level by using fluorochrome-bound monoclonal antibodies (mAbs) to specific activation markers. Currently, two activation markers, CD63 and CD203c, are commonly used for diagnostic purposes. Upon basophil activation, these two markers are commonly upregulated with similar kinetics, but they have distinct characteristics from each other. CD63 has been better validated for drug allergies; however, CD203c is increasingly utilized in recent studies [15, 23, 34, 35, 48, 51, 60]. Upon anaphylactic stimulation, there is degranulation that causes CD63 to appear at the cell surface during the process of fusion of main granules with plasma membranes [2]. Although CD63 is also expressed on platelets, eosinophils, and monocytes; its expression on basophils can be identified using additional stains for basophil markers such as IgE, CD123, CCR3, CRTH2, and CD203c [3]. CD203c can also be used as an identification marker since it is exclusively expressed on basophils, and this expression is related to piecemeal degranulation of basophils [2]. Unlike CD63, CD203c is constitutively expressed on resting basophils at low levels, but it is highly expressed upon activation [99].
Because CD63 and CD203c activation markers do not show the same responses to stimulation, some commercial kits measure both markers simultaneously, to increase the sensitivity of the tests. In some clinical studies, CD203c showed better sensitivity (52%) than CD63 (22%) in patients with amoxicillin allergy [15]; however, other studies reported better sensitivity of CD63 [23, 35]. Another difference between the two markers is their response to IL-3 priming. In commercial kits, IL-3 is often used for increasing BAT sensitivity; its addition results in the enhancement of CD63 expression but a blunted CD203c response to allergen stimulation [3].
For the BAT procedure, fresh whole blood is withdrawn (100 µL per tube) and processed within 4 h, because the basophil reactivity starts to decline after 4 h from sampling [96]. Anti-FcεRI mAb and N-formyl-methionyl-leucyl-phenylalanine (fMLP) are used as positive controls, and stimulation buffer alone as a negative control. If subjects do not respond to anti-FcεRI (called non-responders), then their BAT results cannot be interpreted and have to be rejected for analysis. The response to fMLP is utilized for assessing cellular viability and the ability to express activation markers. Laboratory protocols differ with different commercial kits and between institutions. For diagnostic purposes, researchers may either set up their own in-house protocols, or utilize commercially available BAT kits that are designed to enhance the sensitivity of the tests.
The preparation of drugs and their dose determination is one of the most challenging steps of BAT because they have a narrower range of testing concentrations than inhalant or food allergens [98]. Several varieties of drug allergens are commercially available, but they are expensive, and selection is frequently a limiting factor. In the case of drugs that are not commercially available, dose response curve analyses and cytotoxicity assays are mandatory steps for determination of optimal concentrations [100]. In this section, we have summarized the methods and results from previous studies, as a reference point. Higher drug concentrations can be used for diagnostic purposes since they provide enhanced sensitivity; however, they should be tested in tolerant controls due to the risk of cellular toxicity and nonspecific basophil activation.
Previous dose-response and cytotoxicity studies provided a range of drug concentrations that can be used for stimulation. Beta-lactams, in general, were reconstituted at 0.01, 0.1, and 1 mg/mL in the dilution buffer [15]; and specifically, benzylpenicillin at 0.4 and 2 mg/mL; penicilloyl-polylysine at 0.005 and 0.025 mg/mL; penicillin minor determinant mixture at 0.1 and 0.5 mg/mL; ampicillin at 0.25 and 1.25 mg/mL [14, 16]; clavulanic acid at 0.156 and 0.625 mg/mL [90]; cefuroxime at 0.83 and 1.2 mg/mL; and cefazolin at 0.16 and 0.4 mg/mL [18]. In the case of amoxicillin, 1.25 mg/mL and a range of 0.25-0.31 mg/mL final concentrations were utilized [11, 13, 14].
Several studies successfully tested varying concentrations of NMBAs, ranging from 1:1000 to 1:10 dilutions [21, 23, 25]. At a dilution of 1:10,000, no significant basophil activation was observed [21]. Other studies have reported 5 × 102 µg/mL NMBA concentration as optimal [20]. However, it should be noted that there might be different optimal concentrations required for stimulation [20, 25].
Aspirin intolerance is usually dose dependent; therefore, the dose determination in this case is extremely critical. According to some functional cytotoxicity studies, only diclofenac showed in vitro cytotoxicity at levels higher than 1.25 mg/mL [38]. The concentrations recommended for stimulation are as follows: aspirin at 0.3, 1.25, and 5 mg/mL; paracetamol at 0.3, 1.25, and 5 mg/mL; dipyrone at 0.6, 5, and 20 mg/mL; and diclofenac at 0.08 and 0.3 mg/mL. Interestingly, high concentrations of aspirin (5 mg/mL) enhanced the sensitivity of the test but also lowered its specificity (to 89.5%). Diclofenac at a high concentration (1.25 mg/mL) resulted in false-positive reactions in 36.8% of controls, but it gave acceptable results at lower concentrations. Naproxen at 5 mg/mL resulted in up-regulation of CD63 in controls, giving rise to increased false positives (85.2%); therefore, it was not routinely recommended for use in BAT. The test concentrations determined by other researchers were sometimes quite low [36] but mostly within the range as for aspirin [29-31, 35].
Fluoroquinolones are known to have skin-irritating properties [45]. Recent studies have reported contrasting but interesting results. Two studies have shown negative BAT results in patients. In the first study, 4 patients were administered 1:10, 1:100, and 1:1,000 dilutions of levofloxacin, moxifloxacin, or ciprofloxacin, ranging from 1.6 to 5 mg/mL parenteral preparations [52]; and in the second study, 6 patients were administered ciprofloxacin at 0.05-0.1 mg/mL, levofloxacin at 0.05-0.1 mg/mL, and moxifloxacin at 0.125-0.25 mg/mL [50]. Aranda et al. [49] were the first to report the dose response analyses for fluoroquinolones in a large group of patients (n = 38), and they have provided an optimal stimulation concentration range (ciprofloxacin at 0.2-2 mg/mL; moxifloxacin at 0.1-0.2 mg/mL; and levofloxacin at 2-4 mg/mL) in their subsequent studies [46, 47].
One important point to note is the potential difference in immunogenicity between fluoroquinolones. Researchers have found that in patients with moxifloxacin hypersensitivity, moxifloxacin was the most frequent culprit drug in vivo [47, 49], but it had a lower sensitivity than ciprofloxacin in inducing basophil activation in vitro [49]. These results demonstrated the cross-reactive nature of fluoroquinolone hypersensitivity, and highlighted the involvement of specific critical factors related to in vitro moxifloxacin allergenicity. Recently it was discovered that moxifloxacin underwent photo-degradation, which critically decreased in vitro basophil responses, thus resulting in lower BAT positivity under light (17.9%) than under dark (35.7%) conditions [46]. In contrast, ciprofloxacin did not have different outcomes between light and dark conditions (both 46.4%). It is not confirmed whether these observations are applicable to other kinds of drugs, but they emphasize the importance of accurate drug preparations for conducting in vitro drug assays.
The effects of a wide range of RCM concentrations, from 100 to 105 dilutions, were first tested on 3 × 105 peripheral blood mononuclear cells [57]. Cell viability was measured by staining for annexin-V, and the optimal dilution of RCM was determined to be 1:10 and 1:100. Later studies confirmed the optimal dilutions for RCM at 1:10 [56].
A sufficient number of well-defined cases and controls are necessary for determining appropriate cutoff points for each drug. Based on these, researchers perform receiver operating characteristic (ROC) curve analyses to locate optimal points. However, the prevalence of drug allergies is low, and drug allergens are more varied than inhalant or food allergens.
The cutoff points are usually based on the percentage of activated basophils, e.g., > 15% above background for inhalant or food allergens, and > 10% above background for latex or hymenoptera venoms [41]. However, in the case of drug allergens, the basophil response is usually lower than that of inhalant or food allergens; therefore, the cutoff is set at > 5% above background, or determined specifically for individual drugs. The stimulation index (defined as the percentage of activated basophils after allergen stimulation per negative control stimulation) of ≥ 2 is additionally adopted, to decrease the chances of false positivity resulting from the low cutoff levels.
Leysen et al. [99] recently summarized several factors that should be considered while carrying out the drug BAT. The maximum recommended time interval between the anaphylactic reaction and its testing was 12 months. Effects of medications such as antihistamines and corticosteroids on in vitro basophil reactivity warranted further studies and should be taken into account while testing. Oral intake of 10 mg desloratadine, an antihistamine, did not influence CD63 expression in basophils upon anti-IgE stimulation, even after 3 h. However, a 30-min in vitro pretreatment of basophils with dimethindene (antihistamine) or prednisolone significantly influenced their activation at concentrations 50-fold higher than the therapeutic level, but not at 10-fold higher concentrations [96].
Go to : 
Drug hypersensitivity is an increasingly significant clinical issue; however, diagnosis is difficult because the underlying pathomechanisms are still unclear and allergenic structures are mostly unknown. Although DPT is the gold standard for diagnosis of drug allergies, there are potential risks of systemic reactions. Moreover, polypharmacy frequently confounds identification of the culprit drugs. BAT has several advantages over conventional diagnostic tools; it can assess multiple drugs simultaneously, safely, and specifically. As summarized in this review, BAT is being validated for diagnosing hypersensitivity with beta-lactams, NBMAs, aspirin/NSAIDs, fluoroquinolones, and RCM. In addition, the applications of BAT are rapidly extending into diagnosing allergies caused by various other drugs. In conclusion, we suggest that BAT is a promising diagnostic tool for clinical decisions regarding patients with drug hypersensitivities.
Go to : 
ACKNOWLEDGEMENTS
This study was supported by a grant of Korea Healthcare technology R&D project, Ministry of Health & Welfare, Republic of Korea (A092076).
Go to : 
References
1. Knol EF, Mul FP, Jansen H, Calafat J, Roos D. Monitoring human basophil activation via CD63 monoclonal antibody 435. J Allergy Clin Immunol. 1991; 88:328–338. PMID: 1716273.

2. Macglashan DW Jr. Basophil activation testing. J Allergy Clin Immunol. 2013; 132:777–787. PMID: 23958648.

3. McGowan EC, Saini S. Update on the performance and application of basophil activation tests. Curr Allergy Asthma Rep. 2013; 13:101–109. PMID: 23188565.

5. Aberer W, Bircher A, Romano A, Blanca M, Campi P, Fernandez J, Brockow K, Pichler WJ, Demoly P. European Network for Drug A, hypersensitivity Eigod. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003; 58:854–863. PMID: 12911412.

6. Brockow K, Garvey LH, Aberer W, Atanaskovic-Markovic M, Barbaud A, Bilo MB, Bircher A, Blanca M, Bonadonna B, Campi P, Castro E, Cernadas JR, Chiriac AM, Demoly P, Grosber M, Gooi J, Lombardo C, Mertes PM, Mosbech H, Nasser S, Pagani M, Ring J, Romano A, Scherer K, Schnyder B, Testi S, Torres M, Trautmann A, Terreehorst I. Group EEDAI. Skin test concentrations for systemically administered drugs: an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2013; 68:702–712. PMID: 23617635.
7. Brockow K, Romano A, Blanca M, Ring J, Pichler W, Demoly P. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy. 2002; 57:45–51. PMID: 11991289.

8. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009; 339:b2700. PMID: 19622552.

9. Blanca M, Romano A, Torres MJ, Fernandez J, Mayorga C, Rodriguez J, Demoly P, Bousquet PJ, Merk HF, Sanz ML, Ott H, Atanaskovic-Markovic M. Update on the evaluation of hypersensitivity reactions to betalactams. Allergy. 2009; 64:183–193. PMID: 19133923.

10. Torres MJ, Romano A, Blanca-Lopez N, Dona I, Canto G, Ariza A, Aranda A, Montanez MI, Mayorga C, Blanca M. Immunoglobulin E-mediated hypersensitivity to amoxicillin: in vivo and in vitro comparative studies between an injectable therapeutic compound and a new commercial compound. Clin Exp Allergy. 2011; 41:1595–1601. PMID: 21812844.

11. Torres MJ, Ariza A, Fernandez J, Moreno E, Laguna JJ, Montanez MI, Ruiz-Sanchez AJ, Blanca M. Role of minor determinants of amoxicillin in the diagnosis of immediate allergic reactions to amoxicillin. Allergy. 2010; 65:590–596. PMID: 19968633.

12. Eberlein B, Leon Suarez I, Darsow U, Rueff F, Behrendt H, Ring J. A new basophil activation test using CD63 and CCR3 in allergy to antibiotics. Clin Exp Allergy. 2010; 40:411–418. PMID: 20082620.

13. Garcia-Ortega P, Marin A. Usefulness of the basophil activation test (BAT) in the diagnosis of life-threatening drug anaphylaxis. Allergy. 2010; 65:1204. PMID: 20148801.
14. De Week AL, Sanz ML, Gamboa PM, Aberer W, Sturm G, Bilo MB, Montroni M, Blanca M, Torres MJ, Mayorga L, Campi P, Manfredi M, Drouet M, Sainte-Laudy J, Romano A, Merk H, Weber JM, Jermann TM. Diagnosis of immediate-type beta-lactam allergy in vitro by flow-cytometric basophil activation test and sulfidoleukotriene production: a multicenter study. J Investig Allergol Clin Immunol. 2009; 19:91–109.
15. Abuaf N, Rostane H, Rajoely B, Gaouar H, Autegarden JE, Leynadier F, Girot R. Comparison of two basophil activation markers CD63 and CD203c in the diagnosis of amoxicillin allergy. Clin Exp Allergy. 2008; 38:921–928. PMID: 18331364.

16. Torres MJ, Padial A, Mayorga C, Fernandez T, Sanchez-Sabate E, Cornejo-Garcia JA, Antunez C, Blanca M. The diagnostic interpretation of basophil activation test in immediate allergic reactions to betalactams. Clin Exp Allergy. 2004; 34:1768–1775. PMID: 15544603.

17. Gamboa PM, Garcia-Aviles MC, Urrutia I, Antepara I, Esparza R, Sanz ML. Basophil activation and sulfidoleukotriene production in patients with immediate allergy to betalactam antibiotics and negative skin tests. J Investig Allergol Clin Immunol. 2004; 14:278–283.
18. Sanz ML, Gamboa PM, Antepara I, Uasuf C, Vila L, Garcia-Aviles C, Chazot M, De Weck AL. Flow cytometric basophil activation test by detection of CD63 expression in patients with immediate-type reactions to betalactam antibiotics. Clin Exp Allergy. 2002; 32:277–286. PMID: 11929494.

19. Leysen J, Bridts CH, De Clerck LS, Vercauteren M, Lambert J, Weyler JJ, Stevens WJ, Ebo DG. Allergy to rocuronium: from clinical suspicion to correct diagnosis. Allergy. 2011; 66:1014–1019. PMID: 21375539.

20. Ebo DG, Bridts CH, Hagendorens MM, Mertens CH, De Clerck LS, Stevens WJ. Flow-assisted diagnostic management of anaphylaxis from rocuronium bromide. Allergy. 2006; 61:935–939. PMID: 16867045.

21. Kvedariene V, Kamey S, Ryckwaert Y, Rongier M, Bousquet J, Demoly P, Arnoux B. Diagnosis of neuromuscular blocking agent hypersensitivity reactions using cytofluorimetric analysis of basophils. Allergy. 2006; 61:311–315. PMID: 16436139.

22. Sainte-Laudy J, Boumediene A, Touraine F, Orsel I, Cogne M. Analysis of IgE down regulation induced by basophil activation: application to the diagnosis of muscle relaxant allergic hypersensitivity by flow cytometry. Inflamm Res. 2006; 55(Suppl 1):S21–S22. PMID: 16705374.

23. Sudheer PS, Hall JE, Read GF, Rowbottom AW, Williams PE. Flow cytometric investigation of peri-anaesthetic anaphylaxis using CD63 and CD203c. Anaesthesia. 2005; 60:251–256. PMID: 15710010.

24. Monneret G, Benoit Y, Debard AL, Gutowski MC, Topenot I, Bienvenu J. Monitoring of basophil activation using CD63 and CCR3 in allergy to muscle relaxant drugs. Clin Immunol. 2002; 102:192–199. PMID: 11846462.

25. Abuaf N, Rajoely B, Ghazouani E, Levy DA, Pecquet C, Chabane H, Leynadier F. Validation of a flow cytometric assay detecting in vitro basophil activation for the diagnosis of muscle relaxant allergy. J Allergy Clin Immunol. 1999; 104:411–418. PMID: 10452764.

26. Hagau N, Gherman-Ionica N, Hagau D, Tranca S, Sfichi M, Longrois D. Is a positive history of non-anaesthetic drug allergy a predictive factor for positive allergy tests to anaesthetics? Br J Clin Pharmacol. 2012; 73:460–466. PMID: 21988224.

27. Sudheer PS, Appadurai IR. Anaphylaxis to vecuronium: the use of basophil CD63 expression as a possible screening tool to identify a safe alternative. J Clin Anesth. 2007; 19:555–557. PMID: 18063215.

28. Hagau N, Longrois D, Petrisor C. Threshold for positivity and optimal dipyrone concentration in flow cytometry-assisted basophil activation test. Allergy Asthma Immunol Res. 2013; 5:383–388. PMID: 24179685.

29. Kim MS, Cho YJ. Flow Cytometry-assisted basophil activation test as a safe diagnostic tool for aspirin/NSAID hypersenstivity. Allergy Asthma Immunol Res. 2012; 4:137–142. PMID: 22548206.

30. Abuaf N, Rostane H, Barbara J, Toly-Ndour C, Gaouar H, Mathelier-Fusade P, Leynadier F, Frances C, Girot R. Comparison of CD63 upregulation induced by NSAIDs on basophils and monocytes in patients with NSAID hypersensitivity. J Allergy (Cairo). 2012; 2012:580873. PMID: 22187572.

31. Korosec P, Mavsar N, Bajrovic N, Silar M, Mrhar A, Kosnik M. Basophil responsiveness and clinical picture of acetylsalicylic acid intolerance. Int Arch Allergy Immunol. 2011; 155:257–262. PMID: 21293144.

32. Harrer A, Lang R, Grims R, Braitsch M, Hawranek T, Aberer W, Vogel L, Schmid W, Ferreira F, Himly M. Diclofenac hypersensitivity: antibody responses to the parent drug and relevant metabolites. PLoS One. 2010; 5:e13707. PMID: 21060839.

33. Gomez E, Blanca-Lopez N, Torres MJ, Requena G, Rondon C, Canto G, Blanca M, Mayorga C. Immunoglobulin E-mediated immediate allergic reactions to dipyrone: value of basophil activation test in the identification of patients. Clin Exp Allergy. 2009; 39:1217–1224. PMID: 19400910.
34. Celik GE, Schroeder JT, Hamilton RG, Saini SS, Adkinson NF. Effect of in vitro aspirin stimulation on basophils in patients with aspirin-exacerbated respiratory disease. Clin Exp Allergy. 2009; 39:1522–1531. PMID: 19486029.
35. Bavbek S, Ikinciogullari A, Dursun AB, Guloglu D, Arikan M, Elhan AH, Misirligil Z. Upregulation of CD63 or CD203c alone or in combination is not sensitive in the diagnosis of nonsteroidal anti-inflammatory drug intolerance. Int Arch Allergy Immunol. 2009; 150:261–270. PMID: 19494523.

36. Rodriguez-Trabado A, Camara-Hijon C, Ramos-Cantarino A, Porcel-Carreno SL, Jimenez-Timon S, Pereira-Navarro G, Hernandez-Arbeiza FJ, Fernandez-Pereira L. Basophil activation test for the in vitro diagnosis of nonsteroidal anti-inflammatory drug hypersensitivity. Allergy Asthma Proc. 2008; 29:241–249. PMID: 18534081.

37. Malbrán A, Yeyati E, Rey G, Galassi N. Diclofenac induces basophil degranulation without increasing CD63 expression in sensitive patients. Clin Exp Immunol. 2007; 147:99–105. PMID: 17177968.

38. Sanz ML, Gamboa P, de Weck AL. A new combined test with flowcytometric basophil activation and determination of sulfidoleukotrienes is useful for in vitro diagnosis of hypersensitivity to aspirin and other nonsteroidal anti-inflammatory drugs. Int Arch Allergy Immunol. 2005; 136:58–72. PMID: 15608437.

39. Gamboa P, Sanz ML, Caballero MR, Urrutia I, Antepara I, Esparza R, de Weck AL. The flow-cytometric determination of basophil activation induced by aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) is useful for in vitro diagnosis of the NSAID hypersensitivity syndrome. Clin Exp Allergy. 2004; 34:1448–1457. PMID: 15347380.

40. Gamboa PM, Sanz ML, Caballero MR, Antepara I, Urrutia I, Jauregui I, Gonzalez G, Dieguez I, De Weck AL. Use of CD63 expression as a marker of in vitro basophil activation and leukotriene determination in metamizol allergic patients. Allergy. 2003; 58:312–317. PMID: 12708979.

41. De Weck AL, Sanz ML, Gamboa PM, Aberer W, Bienvenu J, Blanca M, Demoly P, Ebo DG, Mayorga L, Monneret G, Sainte-Laudy J. Diagnostic tests based on human basophils: more potentials and perspectives than pitfalls. Int Arch Allergy Immunol. 2008; 146:177–189. PMID: 18268385.

42. Bosso JV, Schwartz LB, Stevenson DD. Tryptase and histamine release during aspirin-induced respiratory reactions. J Allergy Clin Immunol. 1991; 88:830–837. PMID: 1720795.

43. Steiner M, Harrer A, Lang R, Schneider M, Ferreira T, Hawranek T, Himly M. Basophil activation test for investigation of IgE-mediated mechanisms in drug hypersensitivity. J Vis Exp. 2011; 55:e3263.

44. Blanca-Lopez N, Andreu I, Torres Jaen MJ. Hypersensitivity reactions to quinolones. Curr Opin Allergy Clin Immunol. 2011; 11:285–291. PMID: 21659860.
45. Perez E, Callero A, Martinez-Tadeo JA, Hernandez G, Rodríguez-Plata E, Almeida Z, Garcia-Robaina JC. Are skin tests useful in fluoroquinolone hypersensitivity diagnosis? Ann Allergy Asthma Immunol. 2013; 9. 16. [Epub]. Available from: http://dx.doi.org/10.1016/j.anai.2013.08.012.

46. Mayorga C, Andreu I, Aranda A, Dona I, Montanez MI, Blanca-Lopez N, Ariza A, Nuin E, Blanca M, Miranda MA, Torres MJ. Fluoroquinolone photodegradation influences specific basophil activation. Int Arch Allergy Immunol. 2013; 160:377–382. PMID: 23183272.

47. Blanca-Lopez N, Ariza A, Dona I, Mayorga C, Montanez MI, Garcia-Campos J, Gomez F, Rondon C, Blanca M, Torres MJ. Hypersensitivity reactions to fluoroquinolones: analysis of the factors involved. Clin Exp Allergy. 2013; 43:560–567. PMID: 23600547.

48. Rouzaire P, Nosbaum A, Denis L, Bienvenu F, Berard F, Cozon G, Bienvenu J. Negativity of the basophil activation test in quinolone hypersensitivity: a breakthrough for provocation test decision-making. Int Arch Allergy Immunol. 2012; 157:299–302. PMID: 22041525.

49. Aranda A, Mayorga C, Ariza A, Dona I, Rosado A, Blanca-Lopez N, Andreu I, Torres MJ. In vitro evaluation of IgE-mediated hypersensitivity reactions to quinolones. Allergy. 2011; 66:247–254. PMID: 20722637.

50. Lobera T, Audicana MT, Alarcon E, Longo N, Navarro B, Munoz D. Allergy to quinolones: low cross-reactivity to levofloxacin. J Investig Allergol Clin Immunol. 2010; 20:607–611.
51. Ben Said B, Berard F, Bienvenu J, Nicolas JF, Rozieres A. Usefulness of basophil activation tests for the diagnosis of IgE-mediated allergy to quinolones. Allergy. 2010; 65:535–536. PMID: 19845576.

52. Seitz CS, Brocker EB, Trautmann A. Diagnostic testing in suspected fluoroquinolone hypersensitivity. Clin Exp Allergy. 2009; 39:1738–1745. PMID: 19735271.

53. Yang MS, Lee SH, Kim TW, Kwon JW, Lee SM, Kim SH, Kwon HS, Park CH, Park HW, Kim SS, Cho SH, Min KU, Kim YY, Chang YS. Epidemiologic and clinical features of anaphylaxis in Korea. Ann Allergy Asthma Immunol. 2008; 100:31–36. PMID: 18254479.

54. Kim MH, Park CH, Kim DI, Kim KM, Kim HK, Lim KH, Song WJ, Lee SM, Kwon HS, Park HW, Yoon CJ, Cho SH, Min KU, Kim YY, Chang YS. Surveillance of contrast-media-induced hypersensitivity reactions using signals from an electronic medical recording system. Ann Allergy Asthma Immunol. 2012; 108:167–171. PMID: 22374199.

55. Kim SH, Jo EJ, Kim MY, Lee SE, Kim MH, Yang MS, Song WJ, Choi SI, Kim JH, Chang YS. Clinical value of radiocontrast media skin tests as a prescreening and diagnostic tool in hypersensitivity reactions. Ann Allergy Asthma Immunol. 2013; 110:258–262. PMID: 23535089.

56. Salas M, Gomez F, Fernandez TD, Dona I, Aranda A, Ariza A, Blanca-Lopez N, Mayorga C, Blanca M, Torres MJ. Diagnosis of immediate hypersensitivity reactions to radiocontrast media. Allergy. 2013; 68:1203–1206. PMID: 23991759.

57. Pinnobphun P, Buranapraditkun S, Kampitak T, Hirankarn N, Klaewsongkram J. The diagnostic value of basophil activation test in patients with an immediate hypersensitivity reaction to radiocontrast media. Ann Allergy Asthma Immunol. 2011; 106:387–393. PMID: 21530870.

58. Javaloyes G, Goikoetxea MJ, Sanz ML, Cabrera-Freitag P, Gastaminza G. Basophil activation test in the diagnosis of gadobutrol anaphylaxis. Ann Allergy Asthma Immunol. 2012; 108:286–287. PMID: 22469455.

59. Trcka J, Schmidt C, Seitz CS, Brocker EB, Gross GE, Trautmann A. Anaphylaxis to iodinated contrast material: nonallergic hypersensitivity or IgE-mediated allergy? AJR Am J Roentgenol. 2008; 190:666–670. PMID: 18287437.

60. Hino M, Shimojo N, Ochiai H, Inoue Y, Ando K, Chikaraishi K, Ota S, Okimoto Y, Sunami S, Nakamura R, Teshima R, Sato Y, Kohno Y. Expression of CD203c on basophils as a marker of immunoglobulin E-mediated l-asparaginase allergy. Leuk Lymphoma. 2013; 5. 15. [Epub]. Available from: http://dx.doi.org/10.3109/10428194.2013.794944.
61. Piva E, Chieco-Bianchi F, Krajcar V, Aversa S, Plebani M. Adverse reactions in patients with B-cell lymphomas during combined treatment with rituximab: In vitro evaluation of rituximab hypersensitivity by basophil activation test. Am J Hematol. 2012; 87:E130–E131. PMID: 22987289.

62. Aranda A, Mayorga C, Ariza A, Dona I, Blanca-Lopez N, Canto G, Blanca M, Torres MJ. IgE-mediated hypersensitivity reactions to methylprednisolone. Allergy. 2010; 65:1376–1380. PMID: 20486918.

63. Soriano Gomis V, Perez Sempere A, Gonzalez Delgado P, Sempere JM, Niveiro Hernandez E, Marco FM. Glatiramer acetate anaphylaxis: detection of antibodies and basophil activation test. J Investig Allergol Clin Immunol. 2012; 22:65–66.
64. Apostolou E, Deckert K, Puy R, Sandrini A, de Leon MP, Douglass JA, Rolland JM, O'Hehir RE. Anaphylaxis to Gelofusine confirmed by in vitro basophil activation test: a case series. Anaesthesia. 2006; 61:264–268. PMID: 16480352.
65. Viardot-Helmer A, Ott H, Sauer I, Merk HF. Basophil activation test as in vitro assay for cisplatin allergy. Hautarzt. 2008; 59:883–884. PMID: 18931982.
66. Manso L, Polo B, Fernandez-Nieto M, Sastre LB, del Pozo V, Sastre J. Basophil activation test in a case of systemic hypersensitivity reaction to infliximab with good tolerance to another anti-TNF-alpha agent (adalimumab). J Investig Allergol Clin Immunol. 2010; 20:537–538.
67. Ben Said B, Leray V, Nicolas JF, Rozieres A, Berard F. Methylprednisolone-induced anaphylaxis: diagnosis by skin test and basophil activation test. Allergy. 2010; 65:531–532. PMID: 19839979.

68. Walker AI, Rawer HC, Sieber W, Przybilla B. Immediate-type hypersensitivity to succinylated corticosteroids. Int Arch Allergy Immunol. 2011; 155:86–92. PMID: 21109752.

69. Nucera E, Lombardo C, Aruanno A, Colagiovanni A, Buonomo A, de Pasquale T, Pecora V, Sabato V, Rizzi A, Pascolini L, Ricci AG, Schiavino D. 'Empty sella syndrome': a case of a patient with sodium succinate hydrocortisone allergy. Eur J Endocrinol. 2011; 164:139–140. PMID: 20961968.

70. Lehmann S, Ott H. Glucocorticoid hypersensitivity as a rare but potentially fatal side effect of paediatric asthma treatment: a case report. J Med Case Rep. 2008; 2:186. PMID: 18518974.

71. Bobadilla-Gonzalez P, Perez-Rangel I, Camara-Hijon C, Garcia-Menaya JM, Sanchez-Vega S. Positive basophil activation test result in a patient with acute urticaria induced by cetirizine and desloratadine. Ann Allergy Asthma Immunol. 2011; 106:258–259. PMID: 21354029.
72. Sánchez Morillas L, Rojas Perez-Ezquerra P, Reano Martos M, Sanz ML, Laguna Martinez JJ. Urticaria due to antihistamines. J Investig Allergol Clin Immunol. 2011; 21:66–68.
73. Lee SW, Byun JY, Choi YW, Myung KB, Choi HY. Fexofenadine-induced urticaria. Ann Dermatol. 2011; 23:S329–S332. PMID: 22346270.

74. Caceres Calle O, Fernandez-Benitez M. Allergy to dexchlorpheniramine: study of a case. Allergol Immunopathol (Madr). 2004; 32:306–309. PMID: 15456628.
75. Leysen J, De Witte L, Sabato V, Faber M, Hagendorens M, Bridts C, De Clerck L, Ebo D. IgE-mediated allergy to pholcodine and cross-reactivity to neuromuscular blocking agents: Lessons from flow cytometry. Cytometry B Clin Cytom. 2013; 84:65–70. PMID: 23355309.

76. Dewachter P, Nicaise-Roland P, Kalaboka S, Lefevre J, Chollet-Martin S. Anaphylaxis to amidotrizoate proved by skin testing and flow cytometry-based basophil activation test. Allergy. 2009; 64:501–502. PMID: 19284406.

77. Bensaid B, Rozieres A, Berard F, Bienvenu J, Nicolas JF. IgE-mediated allergy to pristinamycin: the value of skin tests and basophil activation tests. Allergy. 2009; 64:1694. PMID: 19796202.

78. Anders D, Trautmann A. Allergic anaphylaxis due to subcutaneously injected heparin. Allergy Asthma Clin Immunol. 2013; 9:1. PMID: 23305328.

79. Caballero MR, Fernandez-Benitez M. Allergy to heparin: a new in vitro diagnostic technique. Allergol Immunopathol (Madr). 2003; 31:324–328. PMID: 14670287.

80. Hur GY, Hwang EK, Moon JY, Ye YM, Shim JJ, Park HS, Kang KH. Oral muscle relaxant may induce immediate allergic reactions. Yonsei Med J. 2012; 53:863–865. PMID: 22665359.

81. Ebo DG, Piel GC, Conraads V, Stevens WJ. IgE-mediated anaphylaxis after first intravenous infusion of cyclosporine. Ann Allergy Asthma Immunol. 2001; 87:243–245. PMID: 11570623.

82. Manso L, Heili S, Fernandez-Nieto M, Sastre B, Sastre J. Basophil activation in two cases of hydrochlorothiazide-induced noncardiogenic pulmonary edema. Allergy. 2010; 65:135–136. PMID: 19804446.

83. Gamboa PM, Achotegui V, Irigoyen J, Perez-Asenjo J, Merino J, Sanz ML. Hydrochlorothiazide-induced acute non-cardiogenic pulmonary edema. J Investig Allergol Clin Immunol. 2005; 15:299–301.
84. Coors EA, Seybold H, Merk HF, Mahler V. Polysorbate 80 in medical products and nonimmunologic anaphylactoid reactions. Ann Allergy Asthma Immunol. 2005; 95:593–599. PMID: 16400901.

85. Badiu I, Geuna M, Heffler E, Rolla G. Hypersensitivity reaction to human papillomavirus vaccine due to polysorbate 80. BMJ Case Rep. 2012; http://dx.doi.org/10.1136/bcr.02.2012.579.

86. Ebo DG, Bridts CH, Stevens WJ. Anaphylaxis to an urethral lubricant: chlorhexidine as the "hidden" allergen. Acta Clin Belg. 2004; 59:358–360. PMID: 15819380.

87. Cabrera-Freitag P, Gastaminza G, Goikoetxea MJ, Lafuente A, de la Borbolla JM, Sanz ML. Immediate allergic reaction to atropine in ophthalmic solution confirmed by basophil activation test. Allergy. 2009; 64:1388–1389. PMID: 19392999.

88. Dumond P, Franck P, Morisset M, Sainte Laudy J, Kanny G, Moneret-Vautrin DA. Pre-lethal anaphylaxis to carboxymethylcellulose confirmed by identification of specific IgE: review of the literature. Eur Ann Allergy Clin Immunol. 2009; 41:171–176. PMID: 20128230.
89. Philipse E, Sabato V, Bridts C, De Clerck L, Ebo D. Basophil activation in the diagnosis of life-threatening hypersensitivity reaction to iodinated contrast media: a case report. Acta Clin Belg. 2013; 68:140–142. PMID: 23967727.

90. Longo N, Gamboa PM, Gastaminza G, Audicana MT, Antepara I, Jauregui I, Sanz ML. Diagnosis of clavulanic acid allergy using basophil activation and leukotriene release by basophils. J Investig Allergol Clin Immunol. 2008; 18:473–475.
91. Rodriguez Trabado A, Camara Hijon C, Porcel Carreno SL, Rodriguez Martin E, Fletes Peral C, Pereira Navarro G, Jimenez Timon S, Hernandez Arbeiza FJ, Fernandez Pereira L. Anaphylaxis caused by cloxacillin: diagnosis with seriated analysis by way of basophil activation test. Allergy Asthma Proc. 2006; 27:269–272. PMID: 16913272.

92. Renauld V, Goudet V, Mouton-Faivre C, Debaene B, Dewachter P. Case report: perioperative immediate hypersensitivity involves not only allergy but also mastocytosis. Can J Anaesth. 2011; 58:456–459. PMID: 21347740.

93. Monneret G, Benoit Y, Gutowski MC, Bienvenu J. Detection of basophil activation by flow cytometry in patients with allergy to muscle-relaxant drugs. Anesthesiology. 2000; 92:275–277. PMID: 10638929.

94. Chirumbolo S. Basophil activation test in allergy: time for an update? Int Arch Allergy Immunol. 2012; 158:99–114. PMID: 22269476.

95. De Week AL, Sanz ML, Gamboa PM, Aberer W, Bienvenu J, Blanca M, Demoly P, Ebo DG, Mayorga L, Monneret G, Sainte-Laudy J. Diagnostic tests based on human basophils: more potentials and perspectives than pitfalls. II. Technical issues. J Investig Allergol Clin Immunol. 2008; 18:143–155.
96. Sturm G, Kranzelbinder B, Sturm E, Heinemann A, Groselj-Strele A, Aberer W. The basophil activation test in the diagnosis of allergy: technical issues and critical factors. Allergy. 2009; 64:1319–1326. PMID: 19243362.

97. Ebo DG, Bridts CH, Hagendorens MM, Aerts NE, De Clerck LS, Stevens WJ. Basophil activation test by flow cytometry: present and future applications in allergology. Cytometry B Clin Cytom. 2008; 74:201–210. PMID: 18412216.

98. Ebo D, Sainte-Laudy J, Bridts C, Mertens C, Hagendorens M, Schuerwegh A, De Clerck LS, Stevens W. Flow-assisted allergy diagnosis: current applications and future perspectives. Allergy. 2006; 61:1028–1039. PMID: 16918504.

99. Leysen J, Sabato V, Verweij MM, De Knop KJ, Bridts CH, De Clerck LS, Ebo DG. The basophil activation test in the diagnosis of immediate drug hypersensitivity. Expert Rev Clin Immunol. 2011; 7:349–355. PMID: 21595601.

100. Ebo D, Hagendorens M, Bridts C, Schuerwegh A, Stevens W. In vitro allergy diagnosis: should we follow the flow? Clin Exp Allergy. 2004; 34:332–339. PMID: 15005724.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download