INTRODUCTION
The undesired effect that occurs following the administration of a medicine in therapeutic, diagnostic or prophylactic doses is defined as an adverse reaction to medicine (ARM) [
1]. In hospitalized patients, the incidence varies between 1.14% and 14.7% [
2,
3]. From the medicines that have most commonly been associated with ARM, a multicenter study showed antibiotics, followed immediately by contrast media (CM) as the most frequent [
4].
Depending on the susceptibility of the subject, these reactions can be categorized as predictable or unpredictable; the adverse reactions associated with the contrast media (AR-CM) most frequently belong to the second category, in which group, both the immunological and the nonimmunological mechanisms are implicated in generating such reactions [
5].
The incidence of AR-CM depends on the physical-chemical characteristics of the CM, specifically on its osmolality [
6] or ionicity [
7,
8] and the time of commencement of the manifestations [
9,
10], with ample variations that oscillate from less than 1% to more than 30% [
8,
11,
12]. On the other hand, other risk factors that have been associated with the presentation of AR-CM and which stand out, are: a background of previous adverse reactions, the presence of asthma or atopy, suffering some heart alteration, renal insufficiency, old age and the use of medications such as β-blockers, metformin and nephrotoxic agents, among others [
7,
13].
To our knowledge, the pattern of AR-CM has not been investigated extensively in our population; our objective was to estimate the incidence and severity of the AR-CM, in addition to evaluating some possible factors associated with its development.
Go to :

MATERIALS AND METHODS
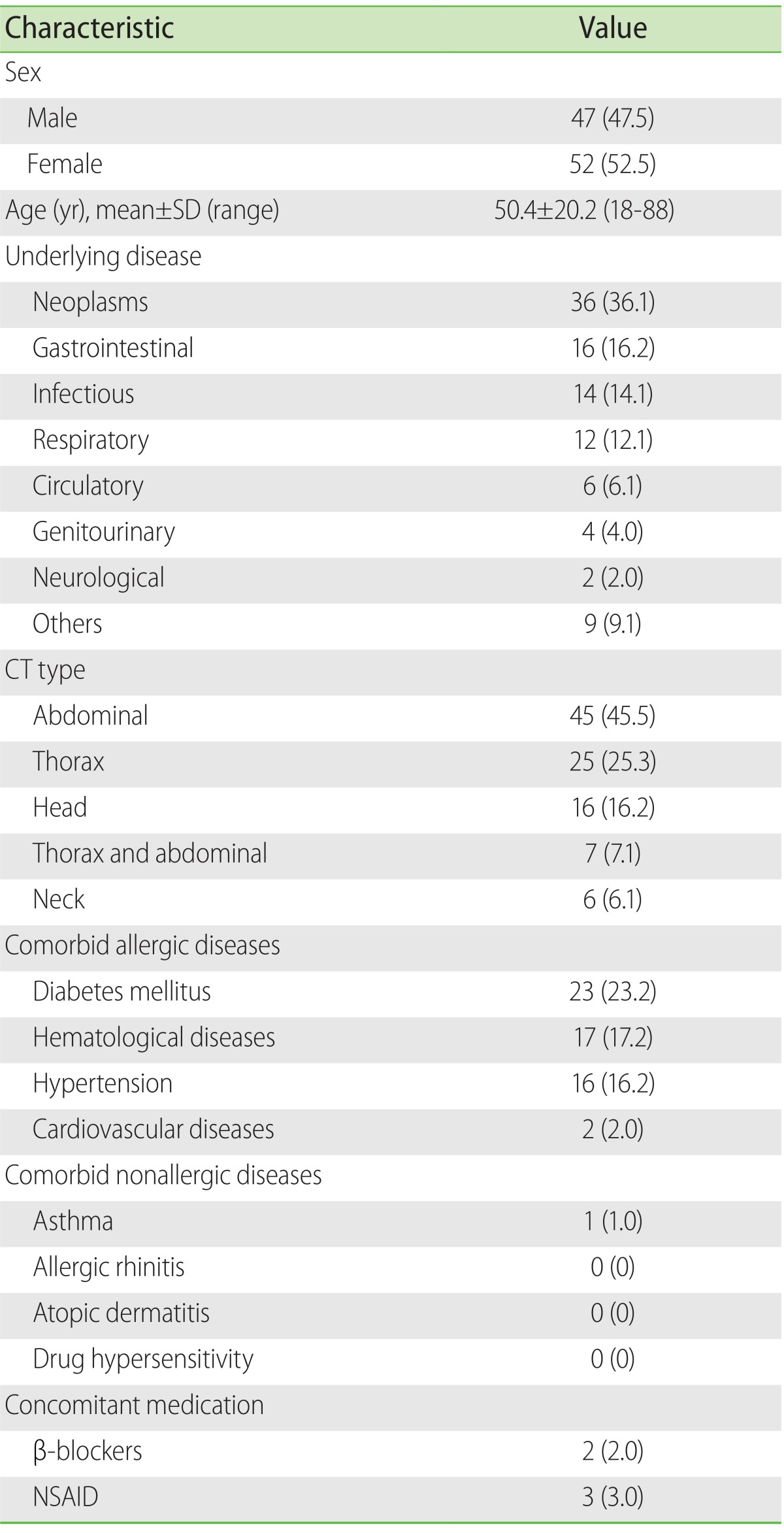
In this observational and longitudinal study, both men and women of 18 years and over were considered, who were hospitalized and on whom a computed tomography with CM (CT-CM) was carried out for the first time in their life. Pregnant women or those who were breast-feeding were excluded and likewise, those subjects with renal insufficiency or baseline serum creatinine levels of ≥1.2 mg/dL; also patients undergoing dialysis or hemodialysis.
The CT was carried out with dual helical multislice tomography (HiSpeed CT/e, General Electric Co., Milwaukee, WI, USA) equipment following protocol standards established in the radiology department, the equipment always being operated by the same radiologist doctor. The same type of monomeric, nonionic, iodinated CM, of a low osmolarity (796 mosm/kg H2O) was used in this study, iopamidol (Iopamiron 300, Bracco SpA, Milan, Italy). The CM was administered intravenously in an automated form, in quantities that varied between 30 and 70 mL depending on the anatomical area to be investigated.
Prior to the data compilation, the Radiology service of our hospital was called on to consult the programming registrations of those patients needing CT-CM, who would fulfill the inclusion criteria. The patients were consecutively recruited and were given a longitudinal follow-up.
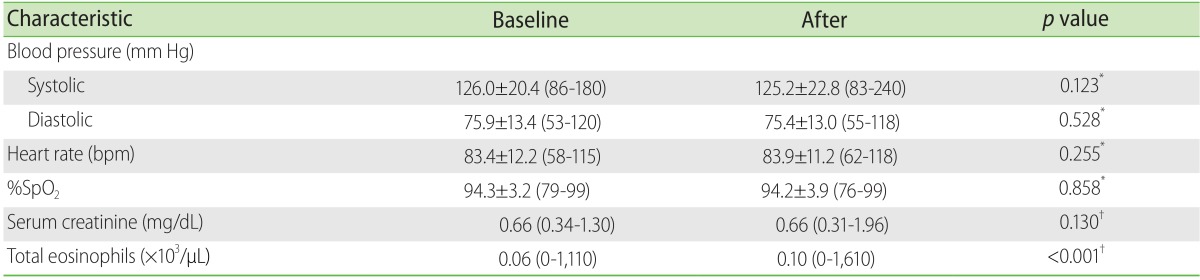
The evaluation for identifying AR-CM was by means of interrogation and physical exploration, all of these being made by a fourth year resident doctor from in the specialty in internal medicine. Each one of the patients was observed at the moment the CM was administered and during the following 60 min; the blood pressure, heart rate, oxygen saturation (measured by a pulse oximeter) was registered before and after the administering of the CM. The nonimmediate reactions were identified 48 h later by means of a second evaluation, identical to the first.
The renal function and alterations in the number of eosinophils were evaluated by means of the quantification of serum creatinine and cell counts, at least 24 h before, and 48 h after the use of the CM.
Definitions
The severity of the AR-CM was defined according to the criteria proposed by the American College of Radiology (Manual on Contrast Media, Version 9, 2013) [
14] as: mild when the symptoms and signs were self limited and with no evidence of progression; moderate, when the discomfort was more pronounced, with focal or systemic affectation; and severe when the clinical alterations were life threatening. For this study, immediate AR-CM was that which occurred within the first 60 min following the administration of CM; after this time, this was considered as nonimmediate [
15]. When the serum creatinine increased from ≥0.5 mg/dL or ≥25% of the previous value in the 3 days following the administration of the CM and in the absence of any other cause that might explain the renal function deterioration, the existence of CM-induced nephrotoxicity (CMIN) was considered absolute and relative, respectively [
16]. A total count of eosinophils in peripheral blood equal to or more than 500 cells ×10
3/µL was considered as eosinophilia. In addition, the atopic comorbidities were registered (asthma, allergic rhinitis, atopic dermatitis, hypersensitivity to medicines) and likewise, the nonatopic comorbidities (diabetes mellitus, hypertension, cardiovascular, and hematological diseases).
Ethics
Prior to the development of the study, all the participants signed a written consent for their participation. Furthermore, approval was obtained on behalf of the Ethics and Investigation Committees prior to the commencement of the investigation.
Statistical analysis
The results are expressed as measures of a central tendency, as considered necessary. In the comparison of means with symmetric distribution, the Student t test was used and in the comparison of medians, the Wilcoxon or Mann-Whitney U rank test was used. In the comparison of proportions, we used the chi-square test, with correction by Yates. All the values of p ≤ 0.05 were considered statistically significant. The IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA) was used for the data processing.
Go to :

DISCUSSION
In our country, this is the first study to provide a more complete description of the behavior of AR following the administration of a CM in a sample of patients hospitalized in a teaching hospital.
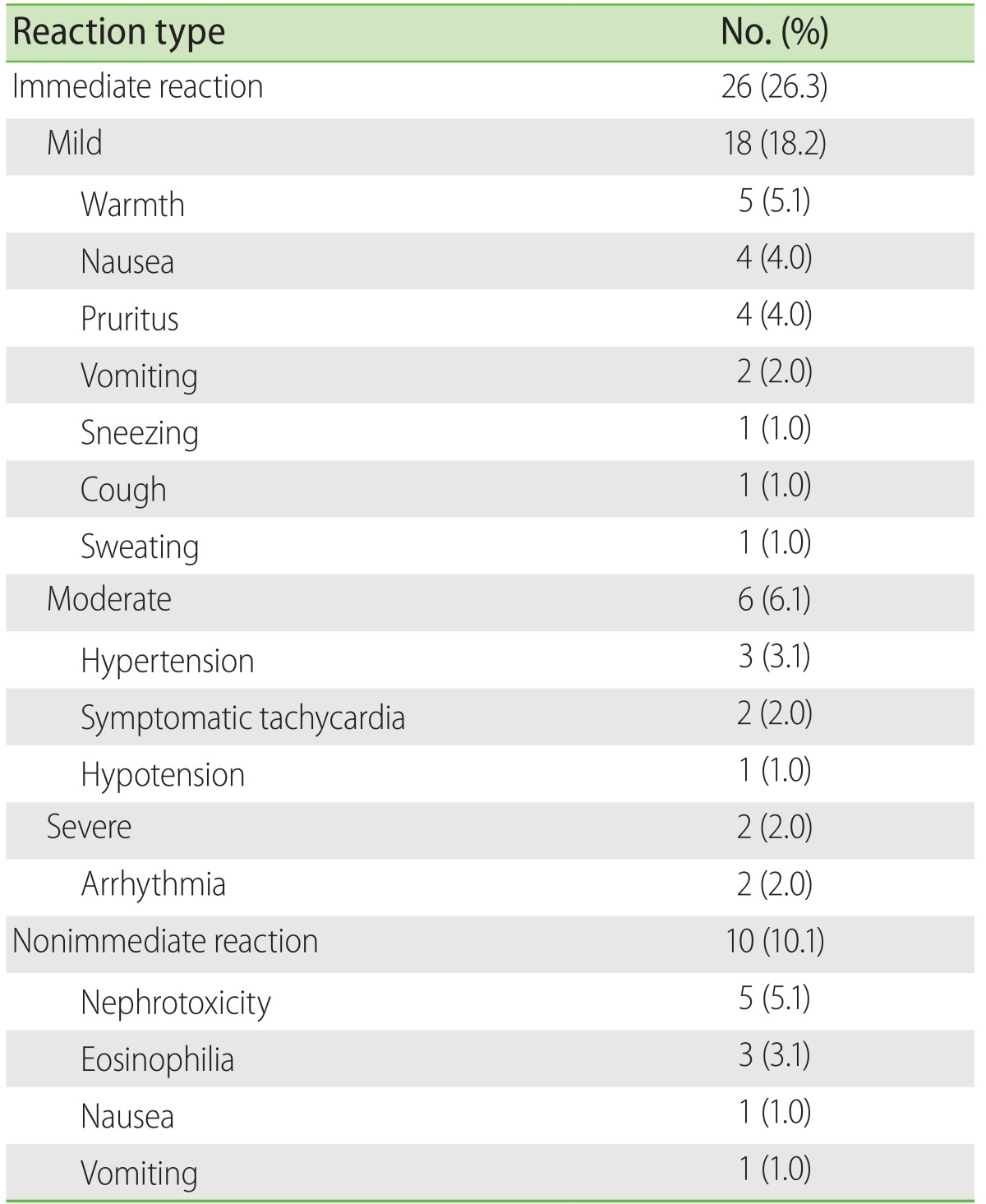
In our study cohort, the incidences of immediate and nonimmediate AR-CM were of 26.3% and 10.1%, respectively. Studies with a methodology comparable with ours, show a rate of acute AR-CM of 34.1% and of delayed reactions of around 50% in a German population [
12]. In an Indian population, Thomas et al. [
7] found that approximately 21% of the patients to whom a CM was administered, showed an immediate reaction. The results of these two studies and our study, notably contrast with those obtained in a multinational postmarketing pharmaco-vigilance study of a nonionic CM, carried out in Europe, Asia, America, and Africa, where the proportion of adverse reactions was of 2.0% [
17]. These differences are even more pronounced when our results are compared with two large studies carried out in the United States, which situated the incidence of adverse reactions at below 1% [
8,
11]. One of the possible explanations of the high incidence of AR-CM in our study was probably the strict longitudinal vigilance that was observed with each patient before, during and after the carrying out of the contrasted study; moreover, a lesser variability was obtained in the quality of the evaluations since these were made by one single qualified researcher and secondary sources such as the Radiology Department records were not used. Other explanations could probably be related to differences of a racial type [
7,
17], or the characteristics of a used, ionic or nonionic CM [
12].
Special mention should be made of the nonimmediate AR-CM. We showed the presence of two alterations that are not usually considered under this heading: eosinophilia and CMIN. Two studies that determined the frequency of nonimmediate AR-CM found that these were of 2.8% and 14.3% [
10,
18,
19]; however, neither of them considered eosinophilia or CMIN as part of this group. In our study, when the symptoms and signs are considered, the incidence of non-immediate AR-CM was 2.0%, but on adding eosinophilia and CMIN, this value increased by up to 10.1%.
A significant increase in the total number of eosinophils was observed after the administering of CM; however, only 3% of the patients fulfilled the definition for eosinophilia. This sub-clinical behaviour was initially been reported by Vincent et al. [
19], who observed that 21% of the patients who have undergone an intravenous urography showed transitory eosinophilia. In a similar manner, Plavsic et al. [
20] documented that 15.5% of the patients to whom a CM was gastro-intestinally administered, manifested eosinophilia. The motives and the consequences of this transitory increase in the quantity of eosinophils are still without explanation. Up until now, the participation of an immunological mechanism is speculated, depending on effector T cells in patients with non-immediate reactions to CM [
21,
22].
When CMIN is defined as an increase in the serum creatinine levels with respect to the baseline levels (absolute CMIN), the incidence in our study was 1.1%; less than in previous studies. In a retrospective study in hospitalized patients, Shema et al. [
23] showed 4.6% of CMIN; a similar value (4.0%) was reported by Caruso et al. [
24]; on the other hand, when the CMIN was defined by a percentage increase ≥25% in creatinine level (relative CMIN), themselves found an incidence of 9.3%, this value contrasting with the 4.1% found in our study. Two other studies that approached the incidence of CMIN, but did not differentiate between absolute and relative CMIN, estimated this at 7.3% and 11.0% [
25,
26]. The differences in the incidences among the different studies are related to the type of definition used for the case, the proportion by which the serum creatinine levels changed with respect to the baseline value and with the moment when the quantification is made [
14]. In our study, the incidence proved to be less, possibly due to the clinical characteristics of the subjects studied, since we decided not to include all those subjects who had previous renal damage or serum creatinine levels of ≥1.2 mg/dL.
An unexpected piece of information in our study, with respect to others, was the absence of cutaneous adverse reactions (urticaria and/or angio-oedema, maculopapular rash) or those related to the extravasation of the CM; we only succeeded in documenting symptoms with cutaneous pruritus or a sensation of warmth, possibly because of the low incidence with which this type of reactions is manifested [
27,
28] or the limited number of subjects that we included. On the other hand, in our hospital, the appropriate collocation of the intravenous catheter in all the patients is verified in a diligent manner, for the purpose of eliminating lesions caused by extravasation of CM.
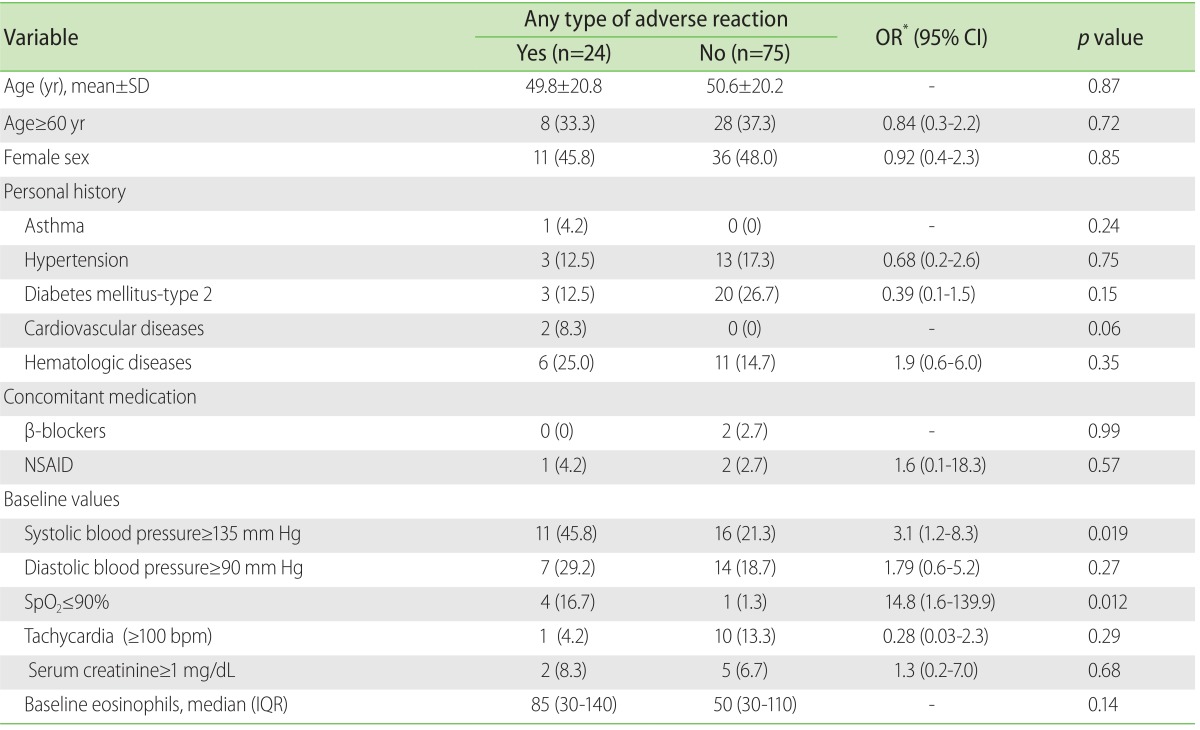
Diverse factors increment the risk of presenting an AR-CM and these would appear to be similar both for the immediate reactions and for the nonimmediate reactions. In a study with 29,508 consecutively recruited patients, Mortele et al. [
8], identified the female sex, a history of allergy, the previous administering of CM and the place of origin of the patients in the out-patients department or emergency services, as important factors for presenting an AR-CM. A multicentric study carried out in Japan [
18] found other factors of association between the AR-CM: history of allergy, previous surgeries, the time of year and the use of concomitant medication. In another study that included 1,131 patients, it was found by means of a structured questionnaire for the detection of AR-CM, that the female sex, psychiatric illness and a history of allergy were very related risk factors [
13].
The risk factors related to the CMIN deserve special mention, where it has been seen that the presence of renal insufficiency, the concomitant use of nephrotoxic medicines (NSAID, aminoglycosides, etc.), diabetes mellitus, dehydration, the age of ≥70 years and heart failure are found to be importantly linked with their presence [
23-
26,
29]. In our study population, the participation of the above-mentioned risk factors could not be documented (no results shown); the same behaviour was observed when the two types of AR-CM were considered as one single type. Again, the explanation can be partially found in the selection criteria that we used, in that we did not consider the subjects with renal insufficiency, with a previous administration of CM or those coming from the department of outpatients; moreover, the quantity of subjects with allergic illnesses was minimum and the medium age of the study group was established at far below 70 years. These same circumstances could have had an important influence on the incidence of AR-CM not being so high.
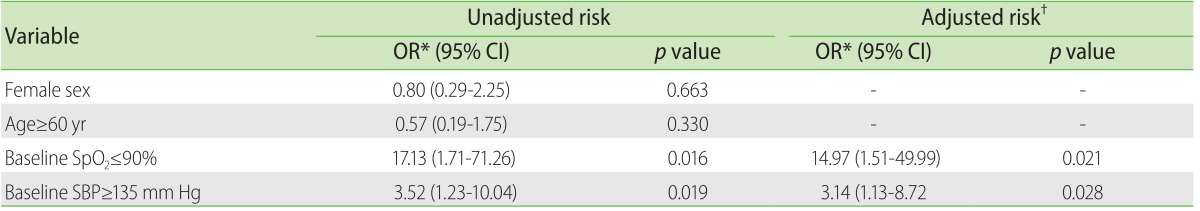
An interesting finding in our investigation that had not been previously reported in other studies was that two biological variables, the baseline systolic blood pressure and %SpO2, were identified as risk factors for presenting any AR-CM. A possible explanation is that those variables could derive from hypertension or heart failure and that this may be the true cause of said modifications; another possibility could be related to the physical-emotional stress that the subjects experience at the moment when the CM is administered, or perhaps the combination of both. Additional studies tending to evaluate the role of the vital functions in the prediction of the AR-CM will help to clarify this phenomenon.
With respect to limitations, our study has several. One of these concerns the limited number, and the lack of randomizing of the subjects studied; however, considering the methodological difficulties represented by the following-up of a group of patients, we consider that our results offer clarity in the behaviour of the AR-CM in the scene of the patients who are hospitalized and with the necessity of a CT-CM. Another limitation concerns the lack of a control group, which would give us the opportunity to make a deeper search for risk factors implicated in the AR-CM. A further limitation was that only one CM, iopamidol, was studied; since in the hospital where the study was made there is no other CM available. Finally, being a study carried out in one sole center, it is not known if this same behaviour occurs in populations with characteristics that are different from ours.
In conclusion, this study of 99 patients on whom a CT-CM was carried out using a medium of a monomeric, nonionic type and of low osmolality, the incidence of AR-CM was greater with respect to previous reports; in general, the majority of these reactions were of an immediate type and of a mild nature. Among the non-immediate AR-CM, because of the clinical implications, the CMIN stands out.
The risk factors that have mostly been reported for the AR-CM, could not be identified in our cohort; in their place, values of high baseline systolic blood pressure and amounts of diminished baseline %SpO2 were significantly related with any type of AR-CM.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download