Abstract
Background
Epinephrine administered intramuscularly is the treatment of choice for anaphylaxis, and more than 1 dose is occasionally required.
Objective
To determine clinical background of anaphylaxis for improving the treatment, management, and prognosis of anaphylaxis.
Methods
Children who had satisfied the diagnostic criteria for anaphylaxis according to the National Institute of Allergy and Infectious Disease Food Allergy and Anaphylaxis Network were selected from our hospital from April 1, 2009 to March 31, 2012.
Results
We analyzed 61 patients from the ages of 2 months to 14 years who satisfied the diagnostic criteria for anaphylaxis. Parents of 32 children (52.5%) reported that they had been administered single dose of epinephrine, and 3 children (4.9%) reported receiving multiple doses of epinephrine. The latter group experienced syncope more often (p = 0.049) than the former and suffered more often from comorbid allergic diseases (p = 0.043) that included either bronchial asthma, allergic rhinitis, or atopic dermatitis. Two (3.3%) children experienced biphasic reactions. Patients who experienced a biphasic reaction were more likely to have experienced syncope (p = 0.033), vomiting (p = 0.02), and administration of multiple doses of epinephrine (p = 0.0016).
"Worldwide, anaphylaxis definitions in common use are 'a serious, life-threatening generalized or systemic hypersensitivity reaction' and 'a serious allergic reaction that is rapid in onset and might cause death' [1]."
Anaphylaxis is not rare and its incidence appears to be increasing, although there are geographic variations. Lifetime prevalence based on international studies is estimated to be 0.05%-2% [2-5]. Unfortunately, estimates suggest that 125-150 deaths occur each year resulting specifically from food-related anaphylaxis [6]. In order to prevent death by quickly recognizing anaphylaxis and to initiate appropriate treatment, it is critically important to acquire a detailed understanding of the clinical characteristics of anaphylaxis.
Epinephrine is the treatment of first choice for anaphylaxis, and guidelines [7, 8] recommend that first responders inject epinephrine intramuscularly, record the time of the dose, and repeat administration in 5-15 minutes, if needed. Published studies [9-17], including those on adults, report that 6%-35% of anaphylactic reactions require multiple doses of epinephrine and have advised that those at risk should carry 2 doses of self-injectable epinephrine.
However, adults and children often demonstrate differences in the clinical presentation of allergic reactions. Moreover, some published studies could either not define anaphylaxis because there was lack of consensus about the definition of anaphylaxis. Only 2 studies have defined anaphylaxis and indicated the risk factors for requiring multiple doses of epinephrine in anaphylactic children [9, 15]. One study is available that describes patients encountered in the emergency department (ED) [9], and another describes the experience in a hospital-based pediatric allergy clinic as well as a private practice-based pediatric food allergy referral clinic [15]. Our hospital has a pediatric allergy clinic as well as an ED; therefore, we were able to investigate more widely clinical characteristics of anaphylactic children.
The primary aim of the present study was to investigate the frequency and risk factors of multiple doses of epinephrine during initial treatment in children with anaphylaxis, with details of both oral food challenges (OFCs) and ED treatment. In addition, we investigated the clinical background of patients and the significance of administering multiple doses of epinephrine.
We conducted a retrospective analysis of the medical records of children who visited Inagi Municipal Hospital from April 1, 2009 to March 31, 2012. All records were selected using the relevant International Classifications of Diseases-tenth revision (ICD-10) diagnostic codes. These codes included allergic urticaria (L50.0), anaphylactic shock due to adverse food reaction (T78.0), other adverse food reactions not elsewhere classified (T78.1), anaphylactic shock unspecified (T78.2), anaphylactic shock due to serum (T80.5), anaphylactic shock due to adverse effects of drugs properly administered (T88.6), and allergy unspecified (T78.4). Investigators reviewed all available medical records for the information as follows: identification of anaphylaxis and its trigger; location (anaphylaxis caused by OFCs in the hospital vs accidental episodes occurring out of the hospital); gender and age; signs and symptoms; treatment; medical history of asthma, atopic dermatitis, or allergic rhinitis; hospitalization; and occurrence of biphasic reactions.
Children were enrolled in the study if the initial clinical presentation met the diagnostic criteria for anaphylaxis [18]. In order to accurately determine the number doses of epinephrine injected during initial treatment of anaphylaxis, we eliminated the doses of epinephrine that were administered through the inhalational route and those administered during biphasic reactions.
OFCs are the gold standard for initial diagnosis of food allergy. All OFCs were performed at our hospital under the supervision of an allergist who was also an investigator in our study. OFCs were performed for outpatients and inpatients, and all OFCs used open challenge methods.
Biphasic reactions were defined as a recurrence of signs and symptoms within 72 hours after resolution of initial remission. Therefore, when patients were discharged, their caregivers were asked to visit or call the hospital in the event of a biphasic reaction within 72 hours.
We used the diagnostic criteria for anaphylaxis outlined by the 2006 Symposium on the Definition and Management of Anaphylaxis, sponsored by the National Institute of Allergy and Infectious Disease Food Allergy and Anaphylaxis Network [18].
This study was approved by the institutional research ethics committee at Inagi Municipal Hospital. The requirement for informed consent was waived by ethics committee because this study only included a review of medical records.
For statistical analyses, SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA) was used. Fisher's exact test was applied to determine differences in proportions, and Mann-Whitney's U test was employed for comparisons of medians. Two-tailed p values < 0.05 were considered to be statistically significant.
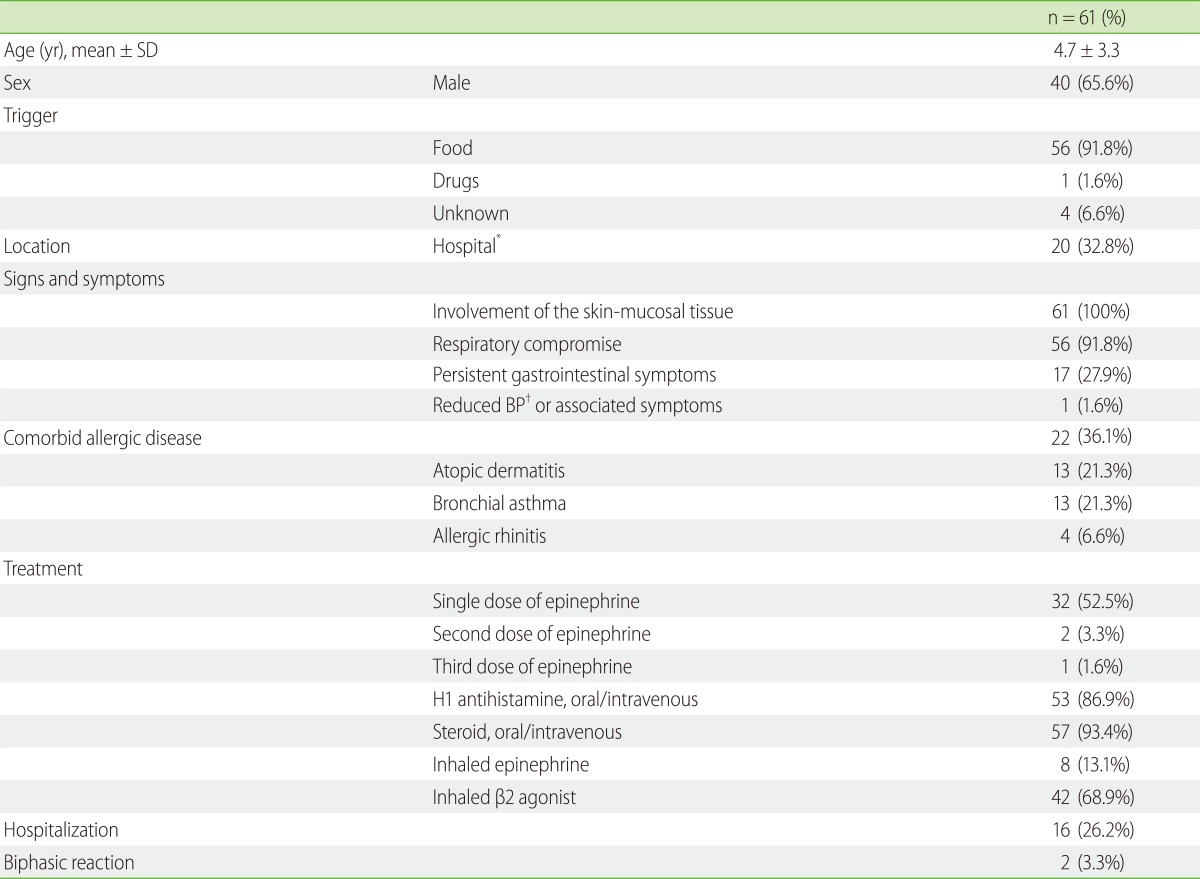
From April 1, 2009 to March 31, 2012, 10,030 children visited our ED, and we performed OFCs on 356 children. Sixty-one children 2 months to 14 years of age satisfied the diagnostic criteria for anaphylaxis at initial clinical presentation. Twenty children (32.8%) met the criteria by OFCs, and the other 41 children (68.2%) made unplanned visits to the hospital for treatment of anaphylaxis caused by ingestion of either a known food allergen or an unidentified allergen.
Of the 61 anaphylactic reactions, foods were the most common trigger with 56 (91.8%) children being affected by them. The cause was unknown for 4 (6.6%) cases, and a drug (aspirin) was responsible for 1 case (1.6%). The top 3 causes of food-induced anaphylaxis were eggs, 23 (41.1%); milk, 12 (21.4%); and wheat, 8 (14.3%) (Table 1).
Thirty-five (57.4%) children with anaphylaxis received epinephrine. Two (3.3%) children received 2 doses and 1 child received 3 doses to resolve the anaphylactic reactions. All these children received 0.01 mg/kg (maximum dose = 0.5 mg) of epinephrine as injections in the lateral side of the thigh. Epinephrine was administered subcutaneously to 2 children, intramuscularly to 33, and inhalationally to 8. Steroids were administered intravenously and orally to 48 and 9 children, respectively. H1-antihistamines were administered intravenously to 45 children and orally to 8. Drugs administered by inhalation were as follows: β2-agonists alone, n = 38; epinephrine, n = 4 children; and β2-agonists simultaneously with inhaled epinephrine, n = 4. One child who received multiple doses of epinephrine was administered both inhalational and intramuscular epinephrine (Table 1).
Sign and symptoms of skin and mucosal involvement were observed in all 61 (100%) children were as follows: generalized hives, n = 59; pruritus or flushing, n = 35; and swollen lips-tongue-uvula, n = 14. The types of respiratory compromise observed in 56 (91.8%) children were as follows: wheeze-bronchospasm, n = 35; dyspnea, n = 6; stridor, n = 5; and hypoxemia, n = 4. Persistent gastrointestinal symptoms were observed in 17 (27.9%) children as follows: vomiting, n = 10 and crampy abdominal pain, n = 9. Decreased blood pressure or associated symptoms including syncope were observed in 1 child (1.6%) (Table 1).
Twenty-two (36.1%) children also suffered from comorbid allergic diseases as follows: atopic dermatitis, n = 13 (21.3%); bronchial asthma, n = 13 (21.3%); and allergic rhinitis, n = 4 (6.6%) (Table 1).
Children treated with multiple doses of epinephrine had syncope (p = 0.049) more often than those treated with ≤ 1 dose. There was no significant difference in the number of doses of epinephrine according to the presence of bronchial asthma (p = 0.52), atopic dermatitis (p = 0.11), or allergic rhinitis (p = 0.19) individually. However, children who have at least one of those bronchial asthma, atopic dermatitis, and allergic rhinitis (p = 0.043) received multiple doses of epinephrine more often. There were no differences in gender, age, involvement of skin or mucosal tissue, respiratory compromise, persistent gastrointestinal symptoms, and location (anaphylaxis caused by OFCs in the hospital vs episodes occurring out of the hospital) (Table 2).
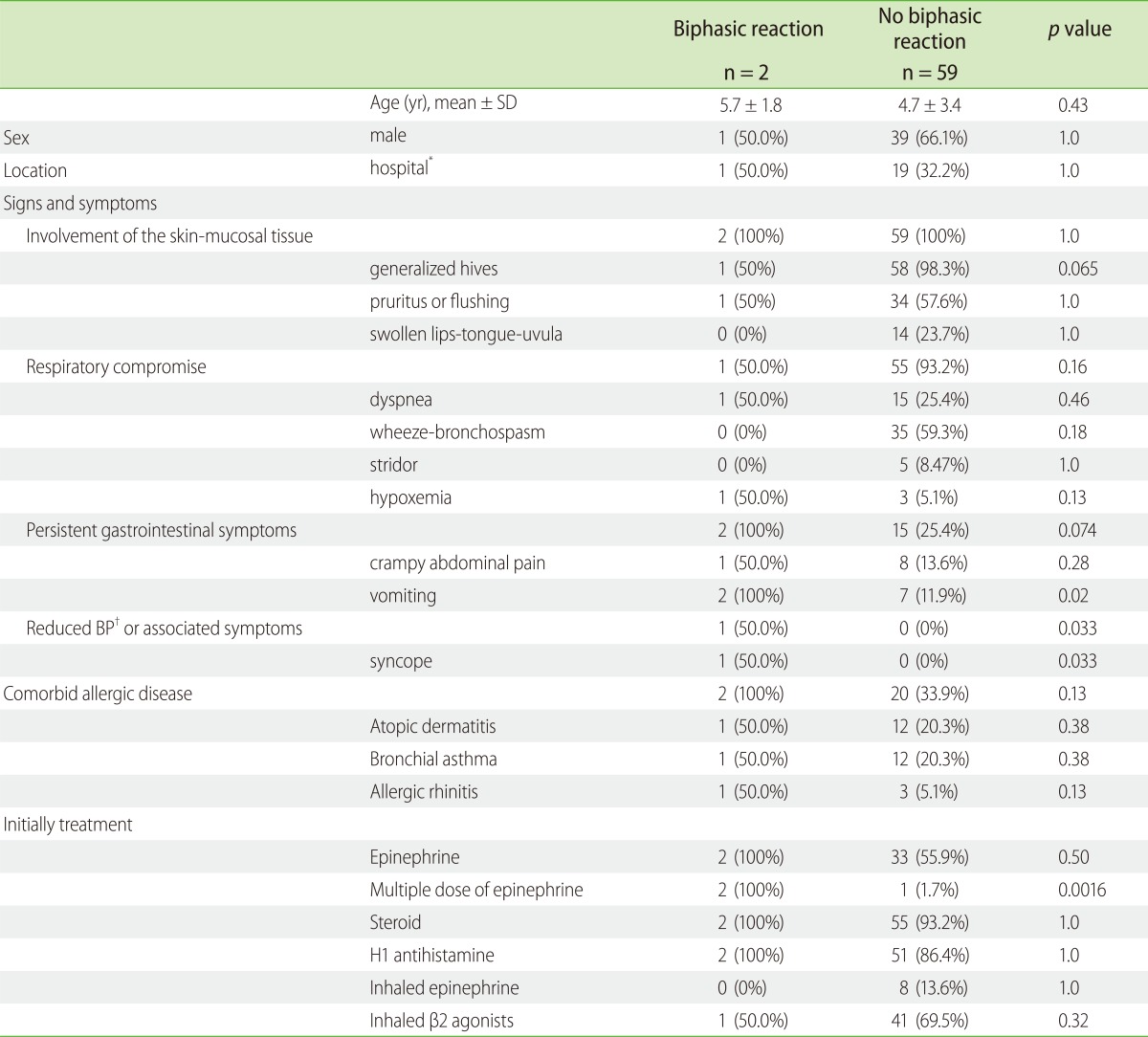
Children suffered biphasic reactions more often when they experienced syncope (p = 0.033) or vomiting (p = 0.02), or when they received multiple dose of epinephrine (p = 0.0016) during initial treatment. There were no significant differences between the occurrence of biphasic anaphylactic reactions and initial treatment with steroids (p = 1.0), H1-antihistamine (p = 1.0), inhalational epinephrine (p = 1.0), and inhalational β2-agonists (p = 0.32) during initial treatment. No children with biphasic reactions were administered inhalational epinephrine (Tables 3 and 4).
Three children were treated with multiple dose of epinephrine; 2 children received 2 doses of epinephrine after OFCs, and 1 child was treated with 3 doses of epinephrine after accidental ingestion. Time interval between the first and second treatment was 12 minutes to 30 minutes, second and third was 40 minutes. Two children experienced biphasic reactions, one of which a 4 years old child who satisfied the diagnostic criteria for anaphylaxis [18] both initial and biphasic reactions (Table 4).
From the data of this study, multiple dose of epinephrine was administered in 3 (4.9%) anaphylactic children and the number of hospitalizations in these children was 16 (26.2%). In addition, biphasic reactions were observed in 2 (3.3%) anaphylactic children.
The "White Book of the World Allergy Organization" [19] provides a definition so this should be acknowledged. However, they also state the following: "There is lack of consensus about the definition of anaphylaxis and this lack of consensus in definition contributes to the variability in its identification, treatment, and the use of epinephrine." When the term anaphylaxis is used, it is necessary to clarify its definition. Therefore, the present study incorporated the diagnostic criteria for anaphylaxis reported by National Institute of Allergy and Infectious Disease and the Food Allergy and Anaphylaxis Network [18].
In all cases reported here, the children seemed to require intramuscular injection of epinephrine. The reason why only 35 (57.4%) children received epinephrine may be explained that there has been lack of knowledge by authorities working in this area. However, there is the lack of consensus regarding when epinephrine should be administered between guidelines [8, 9]. It may affect the reduction of rate of using epinephrine. Moreover, the present study included 41 outpatients who ingested unknown allergens or accidentally ingested a known allergen. We often encounter outpatients with anaphylaxis needlessly treated with epinephrine, because their signs and symptoms improved naturally before arriving at the hospital. However, our analysis yielded almost same rate of epinephrine treatment (57.4%) as those reported by other studies (50%-79%) [9, 10, 17, 18]. However, when investigators reviewed all available medical records, some patients were not administered epinephrine, and two cases had been administrated epinephrine subcutaneously. Therefore, we believe that hospital staff must undergo additional training. Inhalational epinephrine is not effective for treating anaphylaxis. Despite this, it was administered for children with oral swelling or edema because guidelines [8] mention that inhalational epinephrine may be beneficial in such states. However, our data cannot rule out the possibility that inhalational epinephrine provides some benefit by preventing biphasic reactions and the necessity of multiple dose of epinephrine.
Studies of predominantly mixed or adult populations indicate that 6%-35% of anaphylactic reactions due to a variety of causes, require more than 1 dose of epinephrine [9-17]. Studies on children that used the diagnostic criteria for anaphylaxis, found that 5.7%-6.1% received multiple dose of epinephrine [9-17]. We report here that multiple doses of epinephrine were required in 4.9% of cases and are consistent these and other published data [9-17]. Moreover, we emphasize that pediatricians should be aware that multiple doses of epinephrine may be required, and they should reevaluate the administration of epinephrine until the patient's condition stabilizes.
We also report risk factors for multiple doses of epinephrine for children with syncope (p = 0.049) and comorbid conditions of either atopic dermatitis, allergic rhinitis, or bronchial asthma (p = 0.043). Two previous studies indicate that risk factors for repeat epinephrine use in children include advanced age, transfer from a different hospital [9], and asthma [15]. The differences in results between these and our present study may be explained by a number of reasons. In one, Rudders et al. [9] reported that in 74% of cases, subcutaneous epinephrine was administered more frequently. This route of injection has been suggested as a possible explanation for lack of response [20]. In the second study, Järvinen et al. [15] reported only on food allergies in a highly selected patient population suffering from multiple food allergies and receiving more than 1 dose of epinephrine. Therefore, there is a possibility that the findings do not reflect the characteristics of the majority of anaphylactic patients. However, 3 studies, including ours, all report different results. More children population-based studies are required to resolve these discrepancies.
The incidence of biphasic reactions is 6%-11% in children [21, 22]. However the number of documented cases of biphasic reactions in children including those in our study is small. Studies on mixed child and adult populations that have reported incidence of biphasic reaction from 1% to 20% of should be conducted [23]. Many authors recommend, therefore, that patients with an episode of anaphylaxis should be observed carefully for 8-24 hours to monitor for biphasic reactions [23]. However, it is impossible to hospitalize all anaphylactic patients. Therefore, prior to discharge, it is important to inform families of patients about the risk of a biphasic reaction. Because the severity of a biphasic reaction can be greater than the initial reaction, it is important, therefore, to select patients who may be susceptible to a biphasic reaction.
To our knowledge, there are no consistently reported risk factors, but several possible characteristics of the initial episode have been proposed as predisposing factors for a reappearance of symptoms [23]. These factors in children are as follows: > 1 dose of adrenaline, fluid bolus for treatment [22], a delayed administration of epinephrine [21] during the initial event. Moreover, in the present study, we report risk factors for a biphasic reaction that include the initial episode of anaphylaxis followed by multiple treatments with epinephrine (p = 0.0016), syncope (p = 0.033), and vomiting (p = 0.02). We recommend more carefully observation in the hospital for patients presenting with anaphylaxis accompanied by vomiting and syncope, and for patients receiving multiple dose of epinephrine.
We found here that steroids lacked efficacy for preventing biphasic reactions (p = 1.0). However, it is unclear whether corticosteroids given for the primary event can prevent or ameliorate the biphasic reaction [23]. In addition, our data cannot rule out the possibility that steroids provide some benefit in this setting. In contrast, it must be noted that 2 biphasic reactions were experienced by patients receiving prednisone (1 mg/kg/day) after hospitalization. Thus, the administration of steroids cannot completely prevent biphasic reactions, and should not prevent the concurrent use of epinephrine.
Two of three children in our study had been treated with multiple doses of epinephrine and first experienced anaphylaxis at the time of OFCs. Therefore, the patients were provided with self-injectable epinephrine devices to use at the time of onset of anaphylaxis. Some experts recommend carrying 2 doses of self-injectable epinephrine for patients at risk of anaphylaxis [9-11]. In contrast, Järvinen et al. [15] recommended that selection of patients needing 1 or more self-injectable epinephrine requires the identification of risk factors for severe anaphylaxis. We should recommend prescribing 2 devices of self-injectable epinephrine for these 3 patients.
Our study has some limitations. The small sample size may not represent the frequency of the exact causes of anaphylaxis in children. Comparison of anaphylaxis occurring in community settings in the absence of healthcare professionals with a hospital setting where an allergist is available, may affect the accuracy of the number of doses of epinephrine given. Because our study was conducted in Japan and is limited to Japanese subjects, our findings may not be applicable to the healthcare systems in other countries.
The present study analyzed the frequency, predictors of multiple doses of epinephrine in children, and we believe that it provides useful information for the treatment and management of anaphylaxis. For example, we have revealed factors which predispose to the need for multiple doses of epinephrine. In addition, we recommend that children receiving multiple dose of epinephrine should be observed for 24 hours.
References
1. Simons FE, Ardusso LR, Biló MB, El-Gamal YM, Ledford DK, Ring J, Sanchez-Borges M, Senna GE, Sheikh A, Thong BY. World Allergy Organization. World Allergy Organization anaphylaxis guidelines: summary. J Allergy Clin Immunol. 2011; 127:587–593.e1-22. PMID: 21377030.

2. Lieberman P, Camargo CA Jr, Bohlke K, Jick H, Miller RL, Sheikh A, Simons FE. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol. 2006; 97:596–602. PMID: 17165265.

3. Decker WW, Campbell RL, Manivannan V, Luke A, St Sauver JL, Weaver A, Bellolio MF, Bergstralh EJ, Stead LG, Li JT. The etiology and incidence of anaphylaxis in Rochester, Minnesota: a report from the Rochester Epidemiology Project. J Allergy Clin Immunol. 2008; 122:1161–1165. PMID: 18992928.

4. Sheikh A, Hippisley-Cox J, Newton J, Fenty J. Trends in national incidence, lifetime prevalence and adrenaline prescribing for anaphylaxis in England. J R Soc Med. 2008; 101:139–143. PMID: 18344471.
5. Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. J Allergy Clin Immunol. 2009; 123:434–442. PMID: 19117599.

6. Bock SA, Muñoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001; 107:191–193. PMID: 11150011.

7. NIAID-Sponsored Expert Panel. Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, Bahna SL, Beck LA, Byrd-Bredbenner C, Camargo CA Jr, Eichenfield L, Furuta GT, Hanifin JM, Jones C, Kraft M, Levy BD, Lieberman P, Luccioli S, McCall KM, Schneider LC, Simon RA, Simons FE, Teach SJ, Yawn BP, Schwaninger JM. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010; 126:S1–S58. PMID: 21134576.
8. Muraro A, Roberts G, Clark A, Eigenmann PA, Halken S, Lack G, Moneret-Vautrin A, Niggemann B, Rancé F. EAACI Task Force on Anaphylaxis in Children. The management of anaphylaxis in childhood: position paper of the European academy of allergology and clinical immunology. Allergy. 2007; 62:857–871. PMID: 17590200.

9. Rudders SA, Banerji A, Corel B, Clark S, Camargo CA Jr. Multicenter study of repeat epinephrine treatments for food-related anaphylaxis. Pediatrics. 2010; 125:e711–e718. PMID: 20308215.

10. Oren E, Banerji A, Clark S, Camargo CA Jr. Food-induced anaphylaxis and repeated epinephrine treatments. Ann Allergy Asthma Immunol. 2007; 99:429–432. PMID: 18051213.

11. Kelso JM. A second dose of epinephrine for anaphylaxis: how often needed and how to carry. J Allergy Clin Immunol. 2006; 117:464–465. PMID: 16461150.

12. Uguz A, Lack G, Pumphrey R, Ewan P, Warner J, Dick J, Briggs D, Clarke S, Reading D, Hourihane J. Allergic reactions in the community: a questionnaire survey of members of the anaphylaxis campaign. Clin Exp Allergy. 2005; 35:746–750. PMID: 15969665.

13. Banerji A, Rudders SA, Corel B, Garth AM, Clark S, Camargo CA Jr. Repeat epinephrine treatments for food-related allergic reactions that present to the emergency department. Allergy Asthma Proc. 2010; 31:308–316. PMID: 20819321.

14. Webb LM, Lieberman P. Anaphylaxis: a review of 601 cases. Ann Allergy Asthma Immunol. 2006; 97:39–43. PMID: 16892779.

15. Järvinen KM, Sicherer SH, Sampson HA, Nowak-Wegrzyn A. Use of multiple doses of epinephrine in food-induced anaphylaxis in children. J Allergy Clin Immunol. 2008; 122:133–138. PMID: 18547626.

16. Manivannan V, Campbell RL, Bellolio MF, Stead LG, Li JT, Decker WW. Factors associated with repeated use of epinephrine for the treatment of anaphylaxis. Ann Allergy Asthma Immunol. 2009; 103:395–400. PMID: 19927537.

17. Huang F, Chawla K, Järvinen KM, Nowak-Węgrzyn A. Anaphylaxis in a New York City pediatric emergency department: triggers, treatments, and outcomes. J Allergy Clin Immunol. 2012; 129:162–168.e1-3. PMID: 22018905.

18. Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, Brown SG, Camargo CA Jr, Cydulka R, Galli SJ, Gidudu J, Gruchalla RS, Harlor AD Jr, Hepner DL, Lewis LM, Lieberman PL, Metcalfe DD, O'Connor R, Muraro A, Rudman A, Schmitt C, Scherrer D, Simons FE, Thomas S, Wood JP, Decker WW. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006; 117:391–397. PMID: 16461139.

19. Pawankar R, Canonica GW, Holgate ST, Lockey RF. WAO white book on allergy 2011-2012: executive summary [Internet]. 2012. Milwaukee, WI: World Allergy Organization;Available from: http://www.worldallergy.org/publications/wao_white_book.pdf.
20. Simons FE. Apparent lack of response to epinephrine in anaphylaxis. J Allergy Clin Immunol. 2005; 115:640. PMID: 15753921.

21. Lee JM, Greenes DS. Biphasic anaphylactic reactions in pediatrics. Pediatrics. 2000; 106:762–766. PMID: 11015520.

22. Mehr S, Liew WK, Tey D, Tang ML. Clinical predictors for biphasic reactions in children presenting with anaphylaxis. Clin Exp Allergy. 2009; 39:1390–1396. PMID: 19486033.

23. Lieberman P. Biphasic anaphylactic reactions. Ann Allergy Asthma Immunol. 2005; 95:217–226. PMID: 16200811.

Table 2
Comparison of children who were administered multiple dose of epinephrine with those who were not administered multiple dose of epinephrine
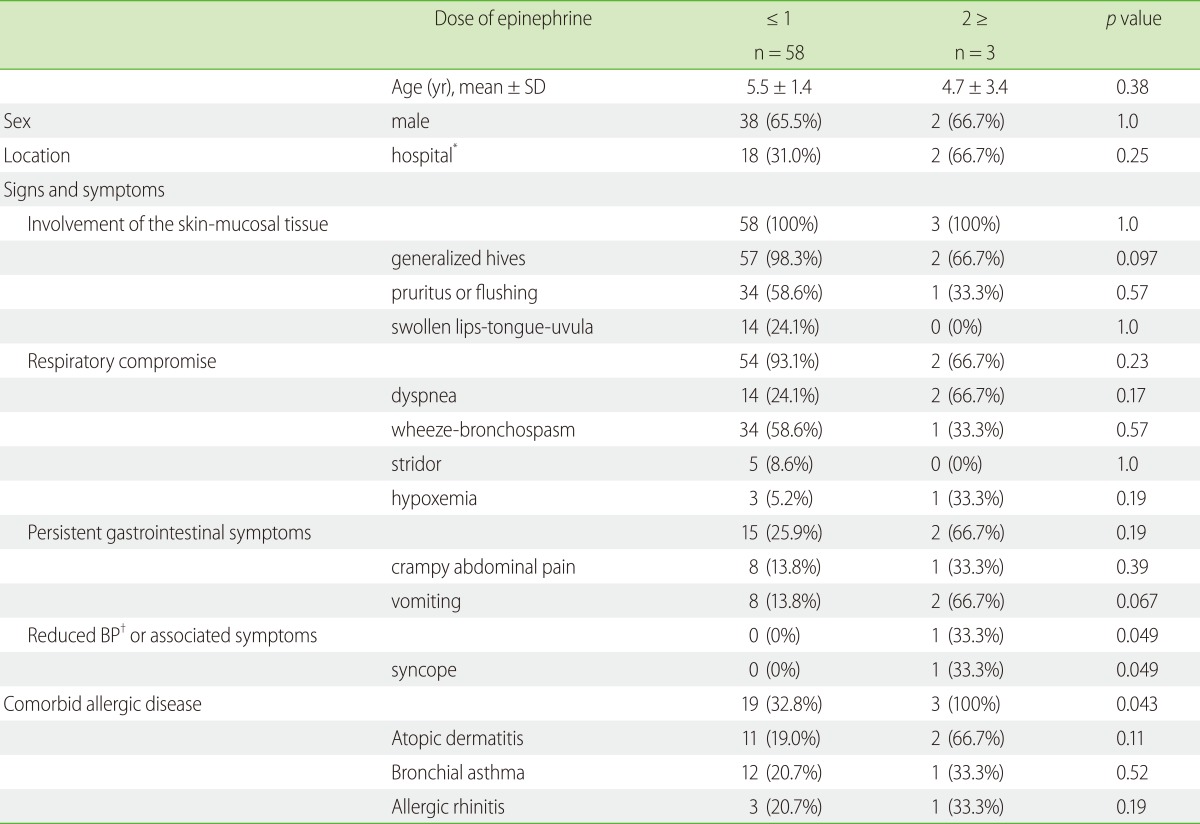
Table 4
Clinical characteristics of 3 patients who were treated with multiple dose of epinephrine
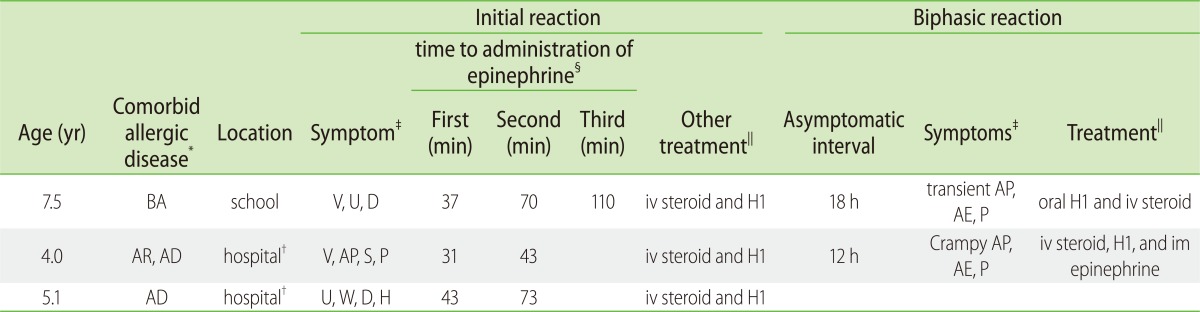
BA: bronchial asthma, AR: allergic rhinitis, AD: Atopic dermatitis, AE: angioedema, AP: abdominal pain, D: dyspnea, H: hypoxia, P: pruritus, S: syncope, U: urticaria, V: vomiting, W: wheeze, H1: H1 antihistamine, iv: intravenous, im: intramuscular
*Comorbid allergic disease
†hospital: oral food challenges
‡symptoms
§first, second, third: time to first or second or third dose from initial symptoms
∥treatment




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download