Abstract
Background
The increasing prevalence of atopic dermatitis (AD) suggests a role for environmental factors in triggering a genetic predisposition in sufferers.
Objective
The purpose of this study was to investigate home environmental factors related to AD severity.
Methods
We conducted a questionnaire survey about the home environmental factors in 380 children from two daycare centers and the Samsung Medical Center outpatient clinic. AD was diagnosed by Hanifin and Rajka's criteria and its severity was assessed by the Severity Scoring of Atopic Dermatitis index. Children were divided into normal control group, mild AD group and severe AD group. Home environmental factors were compared among the three groups and were statistically analyzed using analysis of variance, Chi-square test, Fisher's exact test, and multiple logistic analysis.
Results
Indoor remodeling activities, such as painting (p = 0.004), floor covering (p = 0.001) and wallpaper changing (p = 0.002) were associated with severity of AD. Those in the severe AD group were more likely to live in an apartment (p < 0.001). Severe AD was observed more frequently when the monthly income of household (p = 0.027) and final educational status of mother (p = 0.001) were higher.
Atopic dermatitis (AD) is a chronic, pruritic inflammatory skin disease and is one of the most common allergic diseases in children. The prevalence of AD has recently increased worldwide, with an incidence of 10-20% of children in western countries [1]. In Korea, a nationwide survey conducted among elementary school children revealed that the prevalence of AD increased from 16.6% in 1995 to 24.9% in 2000 [2].
Although AD has been related with genetic factors and family history [3], the rapidly growing incidences of AD in populations lacking any dramatic changes in their gene pools cannot be explained by genetic factors alone; complex causes including both genetic and environmental factors are thought to trigger AD [4, 5]. More exposure to house dust mites following westernization of the home environment was reported to be a risk factor for deterioration of AD [6], another study found that indoor renovation activities seem to be associated with the risk for AD before birth and in the first years of life [7]. The humidity of a child's bedroom and also the uses of a heater or a synthetic pillow were associated with AD symptoms [5]. The presence of indoor gas use, indoor domestic pets, indoor fungi, and environmental exposure to tobacco smoke increase the risk of AD, however, the impacts of all of these factors are still controversial [8, 9].
Although environmental factors are considered to be important risk factors for the deterioration of AD, insufficient data exist for the Korean population. In this study, we attempted to investigate the effects of home environmental factors on the severity of AD using a questionnaire survey.
This study recruited 380 children, including 207 from two daycare centers located in Seoul and 173 patients with AD who visited the Allergy Center of Samsung Medical Center from March 2008 to April 2010. Among these patients, children under the age of 7 years were selected because of age matching with all daycare participants. A pediatric allergist examined each child to diagnose and determine the severity of AD. AD was diagnosed using Hanifin and Rajka's criteria [10], and the severity of AD was determined by using the Severity Scoring of Atopic Dermatitis (SCORAD) index [11]. The participants were divided into three groups: a normal control group without AD, a mild AD group with SCORAD scores less than 20, and a severe AD group with SCORAD scores of 20 and above. The numbers of participants in the three groups were 126 (33.1%), 104 (27.4%) and 150 (39.5%), respectively. This study was approved by the Institutional Review Board of Samsung Medical Center, and it was conducted after gaining written consent from the parents of the participants.
After the purpose and the contents of this study were explained to the participants' parents, self-administered questionnaires were distributed. Questionnaire responses were 91.6% in the normal control group and 93.2% in the AD group. They were asked about birth history (birth weight, gestational age and delivery type), gender, past history and family history of allergic diseases (AD, food allergy, asthma and allergic rhinitis), indoor air quality (parental smoking, frequency of vacuuming and frequency of ventilation), outdoor air quality (distance to nearest major roads and facilities within 2 km of the home), home environment (type of housing, indoor renovations in the last five years and the presence of carpet, fabric sofas, air cleaners, air conditioners, humidifiers, insecticides, and indoor domestic pets), socioeconomic status (monthly family income; low: less than 2 million won, medium: from 2 million won to 5 million won, high: over 5 million won and maternal education), bedding and clothes (type of bedding, frequency of washing, types of detergents used to wash bedding, use of a bleaching agent, and use of fabric softener), water quality, and bathing habits (bathing method, type of bathing water, and frequency of bathing).
The data were collected through a questionnaire survey and physical examination and were statistically analyzed with SAS 9.1 (SAS Institute Inc., USA). We used analysis of variance, Chi-square test and Fisher's exact test to compare differences between risk factors according to AD severity. We obtained the adjusted odds ratios (aOR) and the 95% confidence intervals to analyze the risk factors related with medical history and family history of allergic diseases and home environment; we also revised the potential confounders using multiple logistic regression analysis. Chi-square tests were performed to obtain a p value to analyze home environment risks by AD severity; a p value less than 0.05 was considered to be statistically significant.
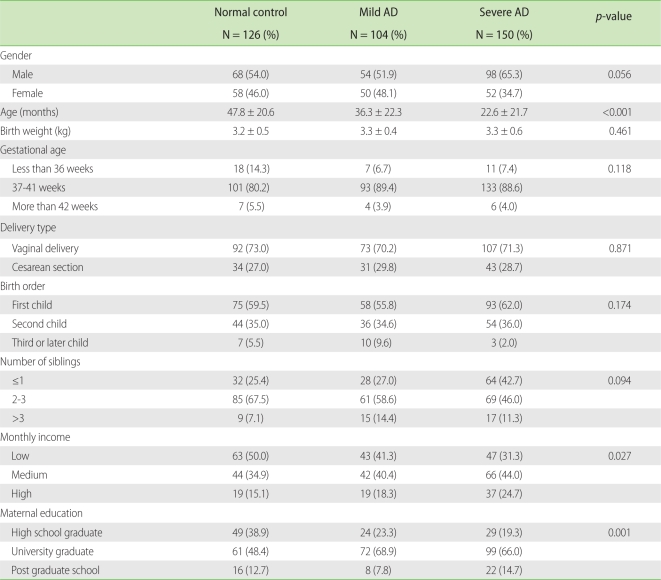
Out of the 380 children (males: 220; females: 160), all three groups had more males than females; however, no significant gender differences among the groups were found. The average ages were 47.8, 36.3 and 22.6 months in the normal, mild AD and severe AD groups, respectively. The severe AD group had the lowest average age of the three groups (p < 0.001). The differences according to birth weight, gestational age, delivery type, birth order, and number of siblings among the three groups were not statistically significant. Severe AD was observed more frequently when the monthly income of household (p = 0.027) and the final educational status of mother (p = 0.001) were higher (Table 1).
The prevalence of food allergy was higher in AD group, and the presence of food allergy significantly increased the risk of mild AD compared to that in the normal control group, with an aOR 3.33 (95% confidence interval (CI) 1.57-7.07) and severe AD compared to that in the normal control group, with an aOR 4.98 (95% CI 2.35-10.54).
Co-morbidity of asthma and/or allergic rhinitis (AR) was the highest in the mild AD group, and the presence of asthma and/or AR significantly increased the risk of mild AD compared to that in the normal control group, with an aOR of 2.20 (95% CI 1.14-4.18). However, a history of asthma did not correlate significantly with AD severity. When we examined the correlation between family history of allergic diseases and AD severity, we found that participants with a family history of allergic rhinitis had a significantly higher risk of mild AD, with an aOR of 1.99 (95% CI 1.14-3.47), but a family history of AD, asthma was not significantly related with the severity of AD (Table 2).
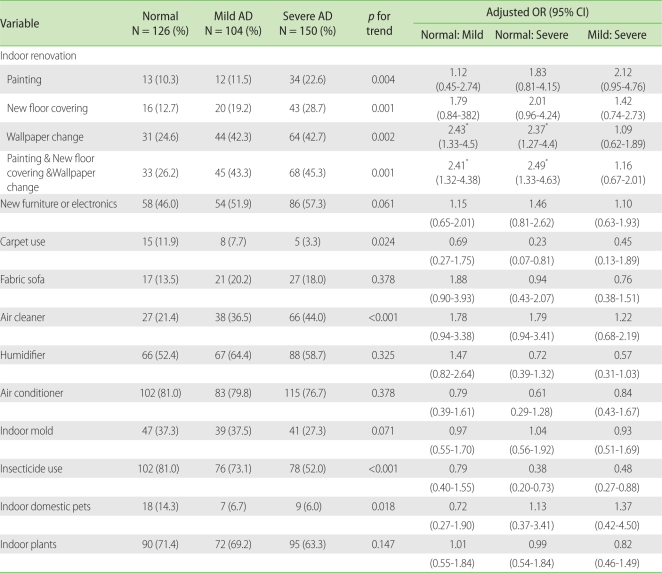
Indoor renovations in the last five years, painting (p = 0.004), floor recovering (p = 0.001) and wallpaper changing (p = 0.002) were associated with a higher severity of AD. The severity of AD was significantly related with having indoor renovations (painting + floor covering + wallpaper changing) activities (p = 0.001). The frequency of carpet use was significantly low (p = 0.024) in the severe AD group, and that of air cleaner use increased with increasing severity of the disease (p < 0.001); fewer participants used insecticides (p < 0.001) and had indoor domestic pets (p = 0.018) as the severity of the disease increased. The severity of AD was not significantly related with having bought new furniture or electronics in the last 12 months or with the presence of fabric sofas, humidifiers, air conditioners, indoor mold, and indoor plants (Table 3).
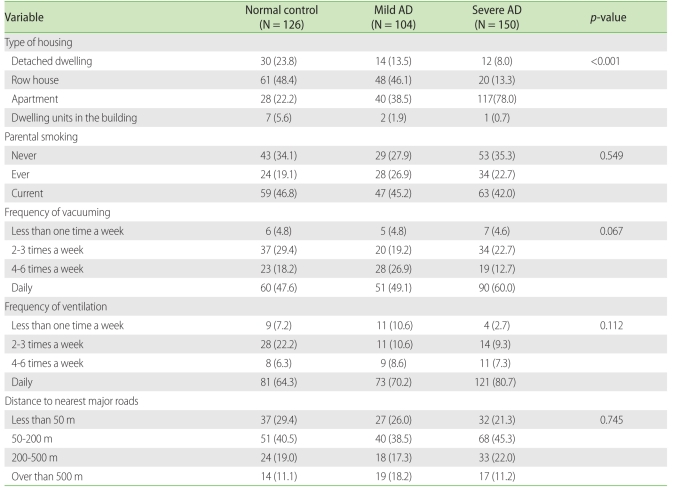
When we investigated the correlation between the types of housing and the severity of AD, we found that most of the normal control group (48.4%) and the mild AD group (46.1%) lived in row houses, while 78.0% of the severe AD group lived in apartments. The differences between the normal and the severe groups (p < 0.001) and between the mild and the severe groups (p < 0.001) were significant. For parental smoking, frequency of vacuuming, frequency of ventilation, distance to major roads with four or more lanes, and presence of facilities around the home did not provoke any significant differences in correlation with AD severity. However, 60.0% and 80.7% of the severe AD group lived in homes that were vacuumed daily or ventilated daily, respectively, and their rates of AD were higher than those of the other two groups having less frequent ventilation and vacuuming (Table 4).
Frequency of washing bedclothes was increased in the severe AD group (p < 0.001), and the use of bleaching agents (p = 0.001) and fabric softeners (p < 0.001) decreased with higher AD severity. For type of bathing water, the participants with higher AD severity tended to use soft water more often than hard water (p < 0.001) (data not shown).
This study showed that some home environmental factors were associated with AD severity. Indoor renovations in the last five years, painting, new floor covering, and wallpaper changing increased AD severity significantly. Our results were consistent with the finding of another study that participants experiencing recent indoor renovation (including painting, floor covering and new furniture) showed a high odds ratios of 1.9 (95% CI 1.4-2.7) for eczema [7]. Current studies on indoor remodeling of buildings and apartments reported that inflammatory response mediators such as interleukin (IL)-8 and monocyte chemoattractant protein-1 in children's blood increased when renovations were conducted [12], that more exposure to indoor volatile organic compounds was related with differentiation of T cells into the Th2 type [13], and that volatile organic compounds crossed the placenta to reach the fetus and then affected the fetal immune system by increasing IL-4 and reducing interferon-γ [14]. In addition, other studies have reported that exposure to volatile organic compounds could damage the epidermal barrier and enhance adverse effect of house dust mite on sensitized subjects with AD [15]. The results of this study were consistent with previous studies which showed that indoor renovations increased the risk for AD and allergic diseases[7, 16]. For the home environment, we found that more patients in the severe AD group lived in apartments; this finding agreed with another study that reported children who moved from detached dwellings to apartments experienced more allergic symptoms in their new environments [17]. According to the 2005 population and housing census data, the number of apartments increased rapidly from 3,455,000 (37.5%) in 1995 to 5,231,000 (47.7%) in 2000 and increased to include more than half of families/individuals by recording 6,616,000 (52.5%) in 2005 in Korea [18]. This change in home environment has been assumed to increase the prevalence of AD and allergic diseases. In this study, we found the association between indoor renovations or living in an apartment with AD severity, but not the causality due to the limitations as a cross-sectional study.
In our study, carpet use, insecticide use and indoor domestic pets were the least frequently found in the severe AD group. Carpet is a source of indoor dust and volatile organic compounds, and the dust in carpet is known to include organic and inorganic compounds, heavy metals and various types of microorganisms [19]. Children living in houses with carpet showed an increased risk of sensitization to house dust mites [20] and a higher risk of asthma or AD [21, 22]. Insecticide exposure has been reported to increase the risk of respiratory and allergic diseases like asthma [23, 24]; however, the correlation between indoor pet exposure and occurrence of allergic diseases is still controversial. Some studies have reported that exposure to indoor pets, such as cats or dogs, within one year after birth declined the risk of sensitization to the allergen and prevented the occurrence of AD [3, 25], while in another study pet allergen exposure increased the risk for AD [26]. However, our findings appeared to show reverse causality. In other words, the parents of the children with severe AD were more likely to avoid potential aggravating factors in their environment. This is supported by the fact that uses of air cleaners and soft water as bathing water were more frequently found in children with severe AD than mild AD [27, 28].
Severe AD was observed more frequently when the monthly income of household and the final educational status of mother were higher. Several studies showed a strong association between parental social class and children's incidence of AD with increasing frequency in privileged classes [29]. It was suggested that cross-infections in early childhood between siblings in large, underprivileged families may play a protective role for the manifestation of AD, and conversely, children of privileged social classes are much more likely to suffer from AD [30].
Our results should be carefully interpreted because of its limitations. First, some changes in home environment were found to be related with AD severity, but their causality was difficult to determine due to the limitations of cross-sectional study. Second, as most of the participants resided in Seoul, their differences in indoor and outdoor environment might not be so large as to result in statistical significance. Third, environmental factors were assessed indirectly through a questionnaire, so in this study there was no direct evidence to prove the relationship between indoor air pollutants and AD severity. Lastly, the questionnaire did not include the question on the final educational status of father. Thus, there might be a bias in evaluating the association between parental education status and AD development. However, lack of information about paternal education status would not skew our conclusion because the final educational status of father is generally similar to or higher than that of mother.
In conclusion, some home environmental factors were associated with the deterioration of AD. In particular, indoor renovations in the last five years and changes in home environment from a detached dwelling to an apartment were associated with AD severity. However, their causality was not clearly elucidated, so further prospective studies are necessary.
References
1. Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008; 121:947–954.e15. PMID: 18155278.

2. Hong SJ, Ahn KM, Lee SY, Kim KE. The prevalences of asthma and allergic diseases in Korean children. Korean J Pediatr. 2008; 51:343–350.

3. Bisgaard H, Halkjaer LB, Hinge R, Giwercman C, Palmer C, Silveira L, Strand M. Risk analysis of early childhood eczema. J Allergy Clin Immunol. 2009; 123:1355–1360.e5. PMID: 19501236.

4. Miyake Y, Ohya Y, Tanaka K, Yokoyama T, Sasaki S, Fukushima W, Ohfuji S, Saito K, Kiyohara C, Hirota Y. Home environment and suspected atopic eczema in Japanese infants: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol. 2007; 18:425–432. PMID: 17617810.

5. McNally NJ, Williams HC, Phillips DR. Atopic eczema and the home environment. Br J Dermatol. 2001; 145:730–736. PMID: 11736896.

6. Oosting AJ, de Bruin-Weller MS, Terreehorst I, Tempels-Pavlica Z, Aalberse RC, de Monchy JG, van Wijk RG, Bruijnzeel-Koomen CA. Effect of mattress encasings on atopic dermatitis outcome measures in a double-blind, placebo-controlled study: the Dutch mite avoidance study. J Allergy Clin Immunol. 2002; 110:500–506. PMID: 12209102.

7. Herbarth O, Fritz GJ, Rehwagen M, Richter M, Röder S, Schlink U. Association between indoor renovation activities and eczema in early childhood. Int J Hyg Environ Health. 2006; 209:241–247. PMID: 16490398.

8. Krämer U, Lemmen CH, Behrendt H, Link E, Schäfer T, Gostomzyk J, Scherer G, Ring J. The effect of environmental tobacco smoke on eczema and allergic sensitization in children. Br J Dermatol. 2004; 150:111–118. PMID: 14746624.

9. Zirngibl A, Franke K, Gehring U, von Berg A, Berdel D, Bauer CP, Reinhardt D, Wichmann HE, Heinrich J. Exposure to pets and atopic dermatitis during the first two years of life. A cohort study. Pediatr Allergy Immunol. 2002; 13:394–401. PMID: 12485314.

10. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh). 1980; 92(Suppl):44–47.
11. Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993; 186:23–31. PMID: 8435513.
12. Herberth G, Gubelt R, Röder S, Krämer U, Schins RP, Diez U, Borte M, Heinrich J, Wichmann HE, Herbarth O, Lehmann I. Increase of inflammatory markers after indoor renovation activities: the LISA birth cohort study. Pediatr Allergy Immunol. 2009; 20:563–570. PMID: 19496969.

13. Lehmann I, Rehwagen M, Diez U, Seiffart A, Rolle-Kampczyk U, Richter M, Wetzig H, Borte M, Herbarth O. Enhanced in vivo IgE production and T cell polarization toward the type 2 phenotype in association with indoor exposure to VOC: results of the LARS study. Int J Hyg Environ Health. 2001; 204:211–221. PMID: 11833293.

14. Lehmann I, Thoelke A, Rehwagen M, Rolle-Kampczyk U, Schlink U, Schulz R, Borte M, Diez U, Herbarth O. The influence of maternal exposure to volatile organic compounds on the cytokine secretion profile of neonatal T cells. Environ Toxicol. 2002; 17:203–210. PMID: 12112628.

15. Huss-Marp J, Eberlein-König B, Breuer K, Mair S, Ansel A, Darsow U, Krämer U, Mayer E, Ring J, Behrendt H. Influence of short-term exposure to airborne Der p 1 and volatile organic compounds on skin barrier function and dermal blood flow in patients with atopic eczema and healthy individuals. Clin Exp Allergy. 2006; 36:338–345. PMID: 16499645.

16. Diez U, Rehwagen M, Rolle-Kampczyk U, Wetzig H, Schulz R, Richter M, Lehmann I, Borte M, Herbarth O. Redecoration of apartments promotes obstructive bronchitis in atopy risk infants--results of the LARS Study. Int J Hyg Environ Health. 2003; 206:173–179. PMID: 12872525.
17. Son KY, Park KS, Hwang HH, Yun BS, Lee SJ, Kim MA, Park JY, Kim KE, Jang KC. Prevalence of allergic diseases among primary school children in Ilsan, Gyeonggi and changes of symptoms after environmental control in 2005. Pediatr Allergy Respir Dis. 2007; 17:384–393.
18. Korea National Statistical Office. 2005 population and housing census. 2005. Daejeon: Korea National Statistical Office.
19. Gravesen S, Larsen L, Gyntelberg F, Skov P. Demonstration of microorganisms and dust in schools and offices. An observational study of non-industrial buildings. Allergy. 1986; 41:520–525. PMID: 3789333.
20. Mahmic A, Tovey ER, Molloy CA, Young L. House dust mite allergen exposure in infancy. Clin Exp Allergy. 1998; 28:1487–1492. PMID: 10024219.

21. Pajno GB, Peroni DG, Barberio G, Pietrobelli A, Boner AL. Predictive features for persistence of atopic dermatitis in children. Pediatr Allergy Immunol. 2003; 14:292–295. PMID: 12911507.

22. Arshad SH, Tariq SM, Matthews S, Hakim E. Sensitization to common allergens and its association with allergic disorders at age 4 years: a whole population birth cohort study. Pediatrics. 2001; 108:E33. PMID: 11483843.

23. Hoppin JA, Umbach DM, London SJ, Henneberger PK, Kullman GJ, Alavanja MC, Sandler DP. Pesticides and atopic and nonatopic asthma among farm women in the Agricultural Health Study. Am J Respir Crit Care Med. 2008; 177:11–18. PMID: 17932376.

24. Fieten KB, Kromhout H, Heederik D, van Wendel de Joode B. Pesticide exposure and respiratory health of indigenous women in Costa Rica. Am J Epidemiol. 2009; 169:1500–1506. PMID: 19372212.

25. Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA. 2002; 288:963–972. PMID: 12190366.

26. Custovic A, Simpson BM, Simpson A, Kissen P, Woodcock A. Effect of environmental manipulation in pregnancy and early life on respiratory symptoms and atopy during first year of life: a randomised trial. Lancet. 2001; 358:188–193. PMID: 11476835.

27. Myatt TA, Minegishi T, Allen JG, Macintosh DL. Control of asthma triggers in indoor air with air cleaners: a modeling analysis. Environ Health. 2008; 7:43. PMID: 18684328.

28. McNally NJ, Williams HC, Phillips DR, Smallman-Raynor M, Lewis S, Venn A, Britton J. Atopic eczema and domestic water hardness. Lancet. 1998; 352:527–531. PMID: 9716057.

29. Werner S, Buser K, Kapp A, Werfel T. The incidence of atopic dermatitis in school entrants is associated with individual life-style factors but not with local environmental factors in Hannover, Germany. Br J Dermatol. 2002; 147:95–104. PMID: 12100190.

30. Snijders BE, Thijs C, van Ree R, van den Brandt PA. Age at first introduction of cow milk products and other food products in relation to infant atopic manifestations in the first 2 years of life: the KOALA Birth Cohort Study. Pediatrics. 2008; 122:e115–e122. PMID: 18595956.

Table 2
Comparison of co-morbidity and family history of allergic diseases in children with AD
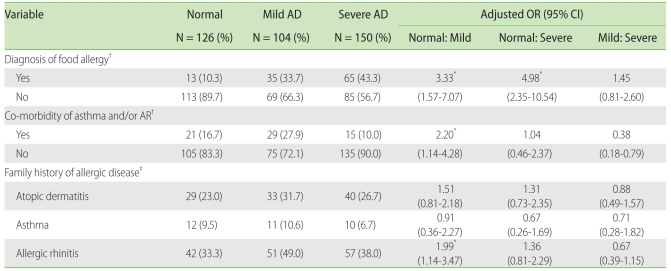
AD, atopic dermatitis; AR, allergic rhinitis; OR, odd ratio; CI, confidence interval. Chi-square test was used to assess the differences among the three groups, and multiple logistic regression analysis was used to assess the relationship between the atopic dermatitis severity participants and each of the parameters. *p < 0.05. †Adjusted by age. ‡adjusted by age, birth weight, parental smoking and socioeconomic status.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download