1. Dixon AE. Rhinosinusitis and asthma: the missing link. Curr Opin Pulm Med. 2009; 15:19–24. PMID:
19077701.

2. Marple BF. Allergic rhinitis and inflammatory airway disease: interactions within the unified airspace. Am J Rhinol Allergy. 2010; 24:249–254. PMID:
20819460.

3. Leynaert B, Neukirch F, Demoly P, Bousquet J. Epidemiologic evidence for asthma and rhinitis comorbidity. J Allergy Clin Immunol. 2000; 106:S201–S205. PMID:
11080732.

4. Huvenne W, van Bruaene N, Zhang N, van Zele T, Patou J, Gevaert P, Claeys S, Van Cauwenberge P, Bachert C. Chronic rhinosinusitis with and without nasal polyps: what is the difference? Curr Allergy Asthma Rep. 2009; 9:213–220. PMID:
19348721.

5. Bachert C, Zhang N, Patou J, van Zele T, Gevaert P. Role of staphylococcal superantigens in upper airway disease. Curr Opin Allergy Clin Immunol. 2008; 8:34–38. PMID:
18188015.

6. Rondón C, Romero JJ, López S, Antúnez C, Martín-Casañez E, Torres MJ, Mayorga C, R-Pena R, Blanca M. Local IgE production and positive nasal provocation test in patients with persistent nonallergic rhinitis. J Allergy Clin Immunol. 2007; 119:899–905. PMID:
17337294.

7. Gevaert P, Holtappels G, Johansson SG, Cuvelier C, Cauwenberge P, Bachert C. Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy. 2005; 60:70–79.
8. Rondón C, Doña I, Torres MJ, Campo P, Blanca M. Evolution of patients with nonallergic rhinitis supports conversion to allergic rhinitis. J Allergy Clin Immunol. 2009; 123:1098–1102. PMID:
19361848.

9. Nicholas B, Djukanović R. Induced sputum: a window to lung pathology. Biochem Soc Trans. 2009; 37:868–872. PMID:
19614609.

10. Rickel EA, Siegel LA, Yoon BR, Rottman JB, Kugler DG, Swart DA, Anders PM, Tocker JE, Comeau MR, Budelsky AL. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008; 181:4299–4310. PMID:
18768888.

11. Bousquet J, Van Cauwenberge P, Khaltaev N. Aria Workshop Group. World Health Organization. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001; 108:S147–S334. PMID:
11707753.

12. van Den Toorn LM, Prins JB, Overbeek SE, Hoogsteden HC, de Jongste JC. Adolescents in clinical remission of atopic asthma have elevated exhaled nitric oxide levels and bronchial hyperresponsiveness. Am J Respir Crit Care Med. 2000; 162:953–957. PMID:
10988112.

13. Kim YK, Chang YS, Lee MH, Hong SC, Bae JM, Jee YK, Chun BR, Cho SH, Min KU, Kim YY. Role of environmental exposure to spider mites in the sensitization and the clinical manifestation of asthma and rhinitis in children and adolescents living in rural and urban areas. Clin Exp Allergy. 2002; 32:1305–1309. PMID:
12220468.

14. Min KU, Kim YK, Park HS, Lee MH, Lee BJ, Son JW, Kim YY, Cho SH. Bronchial responsiveness to methacholine is increased in citrus red mite (Panonychus citri)-sensitive children without asthmatic symptoms. Clin Exp Allergy. 2000; 30:1129–1134. PMID:
10931120.

15. Sohn SW, Lee HS, Park HW, Chang YS, Kim YK, Cho SH, Kim YY, Min KU. Evaluation of cytokine mRNA in induced sputum from patients with allergic rhinitis: relationship to airway hyperresponsiveness. Allergy. 2008; 63:268–273. PMID:
18053010.
16. Di Lorenzo G, Pacor ML, Amodio E, Leto-Barone MS, La Piana S, D'Alcamo A, Ditta V, Martinelli N, Di Bona D. Differences and similarities between allergic and nonallergic rhinitis in a large sample of adult patients with rhinitis symptoms. Int Arch Allergy Immunol. 2011; 155:263–270. PMID:
21293145.

17. Mølgaard E, Thomsen SF, Lund T, Pedersen L, Nolte H, Backer V. Differences between allergic and nonallergic rhinitis in a large sample of adolescents and adults. Allergy. 2007; 62:1033–1037. PMID:
17578499.

18. Durham SR, Gould HJ, Thienes CP, Jacobson MR, Masuyama K, Rak S, Lowhagen O, Schotman E, Cameron L, Hamid QA. Expression of epsilon germ-line gene transcripts and mRNA for the epsilon heavy chain of IgE in nasal B cells and the effects of topical corticosteroid. Eur J Immunol. 1997; 27:2899–2906. PMID:
9394816.
19. Wei CY, Fang SY. Tissue-specific immunoglobulin E in human nasal polyps. Ann Otol Rhinol Laryngol. 2005; 114:386–389. PMID:
15966526.

20. Saitoh T, Kusunoki T, Yao T, Kawano K, Kojima Y, Miyahara K, Onoda J, Yokoi H, Ikeda K. Role of interleukin-17A in the eosinophil accumulation and mucosal remodeling in chronic rhinosinusitis with nasal polyps associated with asthma. Int Arch Allergy Immunol. 2010; 151:8–16. PMID:
19672092.

21. Naclerio RM, Proud D, Togias AG, Adkinson NF Jr, Meyers DA, Kagey-Sobotka A, Plaut M, Norman PS, Lichtenstein LM. Inflammatory mediators in late antigen-induced rhinitis. N Engl J Med. 1985; 313:65–70. PMID:
2582257.

22. Chanez P, Vignola AM, Vic P, Guddo F, Bonsignore G, Godard P, Bousquet J. Comparison between nasal and bronchial inflammation in asthmatic and control subjects. Am J Respir Crit Care Med. 1999; 159:588–595. PMID:
9927377.

23. Braunstahl GJ, Kleinjan A, Overbeek SE, Prins JB, Hoogsteden HC, Fokkens WJ. Segmental bronchial provocation induces nasal inflammation in allergic rhinitis patients. Am J Respir Crit Care Med. 2000; 161:2051–2057. PMID:
10852787.

24. Djukanović R, Lai CK, Wilson JW, Britten KM, Wilson SJ, Roche WR, Howarth PH, Holgate ST. Bronchial mucosal manifestations of atopy: a comparison of markers of inflammation between atopic asthmatics, atopic nonasthmatics and healthy controls. Eur Respir J. 1992; 5:538–544. PMID:
1612155.
25. Semik-Orzech A, Barczyk A, Wiaderkiewicz R, Pierzchala W. Interleukin 17 and RANTES levels in induced sputum of patients with allergic rhinitis after a single nasal allergen challenge. Ann Allergy Asthma Immunol. 2009; 103:418–424. PMID:
19927541.

26. Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, Boulet LP, Hamid Q. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol. 2003; 111:1293–1298. PMID:
12789232.
27. Liu T, Song CH, Liu AM, Xie C, Zhao F, Chen X, Cheng L, Yang PC. Forkhead box P3+ T cells express interleukin-17 in nasal mucosa of patients with both allergic rhinitis and polyposis. Clin Exp Immunol. 2011; 163:59–64. PMID:
21091665.

28. Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, Saito Y, Hatano M, Tokuhisa T, Iwakura Y, Puccetti P, Iwamoto I, Nakajima H. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med. 2008; 178:1023–1032. PMID:
18787221.

29. Louten J, Boniface K, de Waal Malefyt R. Development and function of TH17 cells in health and disease. J Allergy Clin Immunol. 2009; 123:1004–1011. PMID:
19410689.

30. Ciprandi G, Fenoglio D, De Amici M, Quaglini S, Negrini S, Filaci G. Serum IL-17 levels in patients with allergic rhinitis. J Allergy Clin Immunol. 2008; 122:650–651.e2. PMID:
18602680.

31. Ciprandi G, De Amici M, Murdaca G, Fenoglio D, Ricciardolo F, Marseglia G, Tosca M. Serum interleukin-17 levels are related to clinical severity in allergic rhinitis. Allergy. 2009; 64:1375–1378. PMID:
19226302.

32. Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, Van Cauwenberge P, Bachert C. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008; 122:961–968. PMID:
18804271.

33. Barczyk A, Pierzchala W, Sozañska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003; 97:726–733. PMID:
12814161.

34. Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006; 7:135. PMID:
17083726.

35. Zhang N, Holtappels G, Claeys C, Huang G, van Cauwenberge P, Bachert C. Pattern of inflammation and impact of Staphylococcus aureus enterotoxins in nasal polyps from southern China. Am J Rhinol. 2006; 20:445–450. PMID:
16955777.

36. Van Zele T, Gevaert P, Watelet JB, Claeys G, Holtappels G, Claeys C, van Cauwenberge P, Bachert C. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol. 2004; 114:981–983. PMID:
15480349.

37. Islander U, Andersson A, Lindberg E, Adlerberth I, Wold AE, Rudin A. Superantigenic Staphylococcus aureus stimulates production of interleukin-17 from memory but not naive T cells. Infect Immun. 2010; 78:381–386. PMID:
19822653.
38. Wise SK, Ahn CN, Schlosser RJ. Localized immunoglobulin E expression in allergic rhinitis and nasal polyposis. Curr Opin Otolaryngol Head Neck Surg. 2009; 17:216–222. PMID:
19417663.

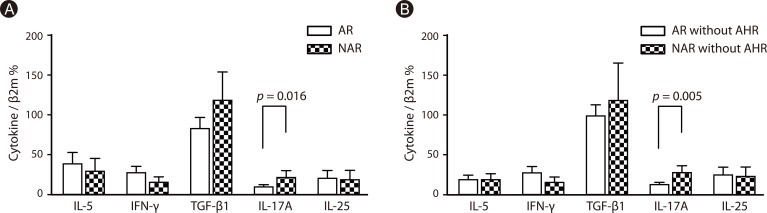
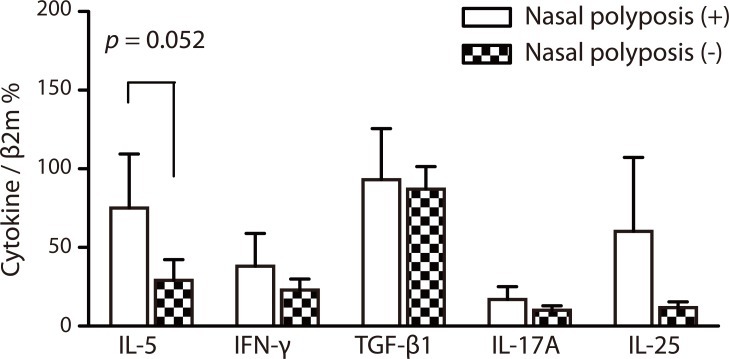




 PDF
PDF ePub
ePub Citation
Citation Print
Print


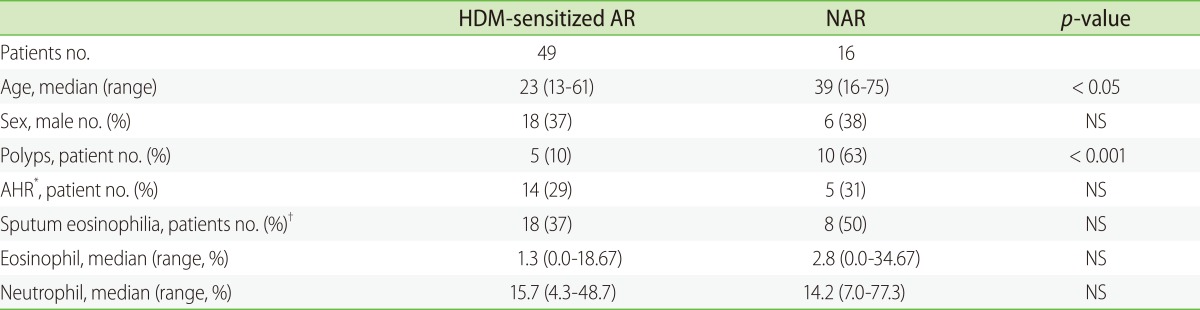
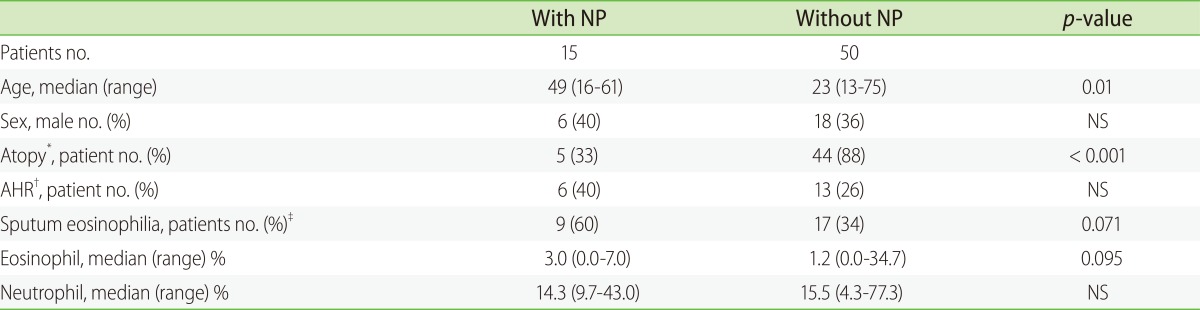

 XML Download
XML Download