Abstract
Background
With use of computed tomography (CT), intravenous contrast media is used routinely to help define anatomy and identify any pathology. Non-ionic iodinated contrast agents have largely replaced ionic agents and although rare, reactions to contrast are still important and more so with the continual increase in CT examinations in the last decade.
Objective
To examine the incidence, severity and risk factors of immediate hypersensitivity reactions to intravenous non-ionic iodinated contrast in CT.
Methods
Data of consecutive patients in an Australian tertiary hospital who developed immediate hypersensitivity reactions to intravenous iopromide during CT were collected and compared with the results of all contrast CTs performed over a four year period. Chi-square statistics and odds ratio are calculated on the variables of age, gender, referral source and seasons of the study.
Results
Forty-seven patients had immediate hypersensitivity reactions of 29,962 patients who underwent contrast CT (0.16%). Thirty-three patients (70%) had a mild reaction, 11 (23%) moderate and three (7%) severe. Sixteen (34%) were male and 31 (66%) were female. Sixty-eight percent were under 55-years of age. Reactions occurred in 0.35% (34 patients) of all outpatients, 0.07% (6 patients) of all emergency patients, and 0.06% (7 patients) of all in-patients. Eighteen (38%) occurred in spring, seven (15%) in summer, 17 (36%) in autumn and five (11%) in winter. There is a statistically significant higher risk of contrast reactions in females (Odds Ratio [OR] 2.41 p = 0.005), patients younger than 55-years old (OR 2.46, p = 0.005), outpatients (OR 5.42, p < 0.001) and CTs performed in spring and autumn (OR 2.77, p = 0.002).
Some years ago non-ionic iodinated contrast agents largely replaced ionic agents, due to their better safety profile, with an immediate hypersensitivity reaction rate of 3.13% compared to 12% with ionics [1]. Some recent studies have reported rates of non-ionic reaction rates from 0.15% [2, 3] to 1.5% [4]. Although rare, immediate hypersensitivity reactions to contrast are still important and more so with the continual increase in computed tomography (CT) examinations in the last decade. A previous large Australian study was published in the 1980s [5] which may not reflect a current experience of contrast reactions. We therefore conduct this study as part of our departmental quality assurance program to examine the incidence, severity and risk factors of such contrast reactions in a large teaching hospital in Australia.
We gained approval for this study by the ethics committee at our institution. All staff working in CT scanning complied with the Radiology Department's policy on the administration of IV contrast. Included in this policy are guidelines and recommendations in identifying and managing adverse reactions to contrast media. Staff members (nursing, radiographers, radiology registrars) were obliged to complete a data sheet of patients who developed immediate hypersensitivity reactions within an hour following intravenous injection of non-ionic iodinated contrast (Iopromide, Ultravist) during CT examinations in the Department of Radiology of a tertiary referral hospital. Due to staffing resource issues, we were not able to undertake longer term follow-up of patients to assess for delayed reactions. The study was undertaken between October 1, 2004 and September 30, 2008. Staff members were also obliged to enter the incident on the Hospital Information System. The collected data included the age, gender, emergency/inpatient/outpatient status, any oral premedications for patients with a prior history of contrast allergy (Prednisone 50 mg 12 hours prior, Prednisone 50 mg + ranitidine 150 mg + cetirizine 10 mg two hours prior to CT), time of study, symptoms, signs and management of the reactions of the patients. Their medical records were reviewed.
The volumes of non-ionic contrast used were between 40-150 mL depending on the scan protocol, patients' renal function and patient size. Because of the nature of referral in a major teaching hospital, with significant all year outpatient services, all types of CT scans were undertaken. Volumes of contrast were, in the main, determined by the study type, e.g. lower with CTs of the brain, higher with CT angiography, CTs of chest, abdomen and pelvis.
The severity of allergy was classified as mild, moderate and severe using the grading system in the ACR Manual on Contrast Media (version 7, 2010) [6].
We also conducted a retrospective search in the hospital electronic database of all the patients who received the same contrast in CT during the same period. Data including age, gender, emergency/inpatient/outpatient status, time of study of the patient were collected. In Australia, Spring is defined as September to November; Summer, December to February; Autumn, March to May and Winter, June to August [7]. We excluded from our patient cohort unconscious, intubated or sedated patients from A&E because identification of immediate, adverse reactions would have been difficult in this small, critically ill, group of patients.
Characteristics of patients who developed immediate hypersensitivity reactions following intravenous injection of non-ionic iodinated contrast during CT examination and those who did not are presented using descriptive statistics. Categorical data (e.g. gender, age group, referral source, season of admission) were described proportionally and Chi-square statistics was used to test a significant association between the contrast reaction group and these categorical exposure variables. To quantify a magnitude of the association, odds ratios (ORs) were calculated, Cornfield's 95% confidence limits for ORs were calculated. Chi-square statistics was corrected for continuity where appropriate, in that case Yates corrected Chi-square was used. Analyses were performed using SPSS statistical software for Windows version 17.0 and EpiInfo software. The p-value of 0.05 was taken as statistically significant.
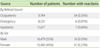
A total of 47 from 29,962 patients who underwent contrast enhanced CT studies (CE-CT) developed immediate hypersensitivity reactions to contrast (CR) (0.16% or 1 in 625 patients). There were no variations in the types of CT scans, and therefore no variations in proportions of patients with higher or lower doses of iodine contrast, between the seasons or the years. Table 1 denotes the number of patients, and rates of reactions, from the varied referral sources and by sex segregation.
The age range in the CR group was 16 to 81-years with a mean of 45.6-years compared with an age range of 12 to 106-years and a mean of 56.7-years in the CE-CT group. Figure 1 shows the number of patients in the CR and CE-CT group by age bands. The peak in CR was in the 50 to 59-year band as against the peak in CE-CT in the 60 to 69-year band. There were a higher proportion of patients under 55-years of age in the CR group (68%) than in the CE-CT group (46%).
Of those patients with CR, six (13%) were emergency department patients, seven (15%) were inpatients and 34 (72%) were outpatients. In the CE-CT group, the percentages were 28%, 39%, and 33% respectively.
In the CR group, 18 (38%) occurred in spring, seven (15%) in summer, 17 (36%) in autumn and five (11%) in winter. The proportion was 25%, 24%, 26%, and 25% respectively in the CE-CT group (Fig. 2). Figure 3 shows the number of patients in the CR and CE-CT groups by month, with peaks in the CR group in April and October and troughs in February and August.
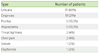
Table 2 summarises the types of reactions recorded. All the listed reactions were recorded, irrespective of degree, including any mild urticaria or pruritis. Flushing was not specifically recorded, because this is a common experience by patients given IV iodinated contrast. A few patients experienced more than one of the reactions listed. Three patients had angioedema and urticaria; one had dyspnea and urticaria; one had dyspnea and diaphoresis; and one had angioedema, dyspnea, seizure and urticaria. Thirty-three (70%) had a mild reaction, 13 (28%) moderate and one (2%) severe. No fatality was recorded. Fourteen (30%) had prior contrast medium exposure with no history of contrast allergy and two (4%) had mild reactions despite pre-medication for prior contrast allergy. Twelve (25%) had a history of allergy other than to contrast medium, two (4%) of these patients also had a history of allergic rhinitis (one in spring and one in winter). A total of six (13%) had a history of asthma (four in spring or autumn, two in winter), with four of these patients also having allergy other than to contrast medium.
The following factors were found to have a statistically significant higher risk of contrast reactions: 1) females, OR 2.41 (1.27-4.6), p = 0.005; 2) when younger than 55 years old, OR 2.46 (1.29-4.75), p = 0.005; 3) outpatients, OR 5.42 (2.76-10.82), p < 0.001); 4) Spring and autumn, OR 2.77 (1.39-5.64), p = 0.002. When the eight patients in the CR group with asthma or allergic rhinitis were removed, the OR for spring and autumn is 3.17 (1.44-7.17), p = 0.002.
Our study of 29,962 patients has a relatively low incidence of 0.16% immediate hypersensitivity reactions to non-ionic contrast. This is similar to the 0.15% reported in the largest single series to date of 298,491 patients using the same non-ionic contrast agent, Iopromide, by Hunt et al. [3], but is lower than many other large series in the literature, Wang et al. [2] (0.6%, 84,928 patients), Mortelé et al. [8] (0.7%, 29,508 patients), Cochran et al. [9] (0.7%, 73,039 patients) and the landmark Japanese series, Katayama et al. [1] (3.13%, 168,363 patients). However, the proportion between mild (70%), moderate (28%) and severe (2%) reactions in our study are comparable to these studies [2, 3, 8]. Our incidence of severe reaction of 0.01% is lower than reported by these series. No fatality was recorded and this is in keeping with the low mortality rate of one in 100,000 [10] to almost one in 300,000 [3].
Female patients in our study had a significant higher rate of reaction than men. Other studies have also observed this higher rate in females [2, 3, 8, 4, 11].
Our study had a significantly higher rate of reaction in less than 55-year-old and the single severe reaction occurred in a patient less than 30-years of age. Whilst some series did not observe a higher risk with younger age [1, 8], Kopp et al. [4] had listed the highest risk age group to being the 18-30-years old, with a reported rate of 1.9%. This figure is somewhat higher than our 0.28% (<30 years old) but Kopp et al. [4] also included non-allergic reactions such as heat sensation and metallic taste. A large paediatric series (less than 21-years old) with 12,494 patients by Callahan et al. [12] showed a rate of 0.46% with significant risk of adverse reaction with increasing age up to 18-years of age.
Outpatients in our study also had a significantly higher risk of developing an immediate reaction when compared to inpatients and emergency patients. Mortelé et al. [8] was the first study to observe this outpatient dominance (over 70% of all immediate reaction) and postulated that the difficulty in accessing clinical information in outpatients and perhaps that outpatients had more risk factors that were unknown may have contributed to this finding.
To the best of our knowledge, no prior repor ts have demonstrated a significant relationship between the incidence of immediate reaction to monthly and seasonal times. This factor was examined recently by Mortelé et al. [8] and Callahan et al. [12] with both studies finding no significant correlation. Seasonal variations to non-immediate or delayed reaction to contrast was observed in a study of 4,875 patients by Mikkonen et al. [13], which showed a higher incidence in the Northern Hemisphere summer months (April to June) suggesting a photosensitive effect. Our series is the first to observe a significant higher incidence of immediate reactions in spring and autumn. A previous Australian series with 30,268 patients (non-ionic contrast subgroup) by Palmer [5] did not examine the seasonal factor. Other potential relevant variables such as asthma and allergic rhinitis did not seem to confound our results as removing these patients from our CR data still yielded a significant result demonstrating peak incidences in spring and autumn. It may be that patients should be less susceptible to contrast allergy during those months, as they are likely to be on increased dose of B2 agonist and/or steroids inhalers. However, it is also possible that patients who have greater symptoms in "allergy seasons" may have more reactive mast cells, and it is possible that many allergic patients be under medicated. We are therefore uncertain as to whether this seasonal variation is limited to our centre or whether this might be an unrecognized geographical risk factor seen in Australia or Southern Hemisphere. A wider study may be valuable in further evaluating this recorded phenomenon. Our seasonal variation is also not explained by any seasonal variations in types of scans, or levels of iodinated contrast given, because there was no variation in our CT referral patterns.
Although immediate allergic reactions to contrast media are often considered non-IgE-mediated, in a report by Brockow et al. [14] up to 50% of their patients had a positive skin test 2-6 months later after the contrast reaction, supporting the concept of an IgEmediated reaction, with histamine release from basophils and mast cells [15]. In patients with negative skin test, the mechanism remains speculative and histamine release can also be due to i) direct membrane effect from the osmolarity or chemical structure (non IgE-mediated) ii) complement activation or iii) direct bradykinin formation [16].
It is also of note that among our patients who had reactions to contrast, 30% had prior contrast exposure with no history of contrast allergy and 4% had mild reactions despite pre-medication for prior contrast allergy. The former figure is similar in Katayama et al. [1] and the latter figure is 7% in Hunt et al. [3], prophylactic use of pre-medication is still debated and the recurrence rate of contrast reaction after corticosteroids premedication has been estimated to be around 10% [17].
There are several limitations to our study. This is a prospective collection, but retrospective data analysis study at a single institution using a single branded non-ionic contrast agent, Ultravist (Iopromide) that may not be generalised to other non-ionic agents or centres. We did not collect data on the incidence of asthma and allergic rhinitis in the CE-CT group and the diagnosis of such in the CR group is relied on from the medical records. We did not refer our patients with immediate reactions to any follow-up skin tests. Analysis of a prior allergy history and prior exposure to contrast was limited to the 47 patients with reactions, and was not evaluated in the other, over 29,900 patients. Therefore we are not able to define the levels of risk in all our patients.
In conclusions, the rate of immediate hypersensitivity reactions to intravenous non-ionic iodinated contrast in CT examination is low and most of these reactions are mild, with a significant higher incidence in females, patients younger than 55 years of age, outpatients and CT examinations performed in Spring and Autumn. This seasonal variation is not reported previously in the literature with any definable cause being elucidated from this study. Further studies will be needed, especially data from other Australian institutions, to determine whether this is an unrecognized geographic risk factor.
Figures and Tables
 | Fig. 2Comparison of contrast reactions (CR) and contrast enhanced CT studies (CE-CT) by Australian seasons. |
ACKNOWLEDGEMENTS
The authors are grateful to Ms Pamela Dougan, Research Assistant, Department of Radiology, Westmead Hospital, Australia for her assistance in preparing and proof-reading the manuscript.
References
1. Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990. 175:621–628.

2. Wang CL, Cohan RH, Ellis JH, Caoili EM, Wang G, Francis IR. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am J Roentgenol. 2008. 191:409–415.

3. Hunt CH, Hartman RP, Hesley GK. Frequency and severity of adverse effects of iodinated and gadolinium contrast materials: retrospective review of 456,930 doses. AJR Am J Roentgenol. 2009. 193:1124–1127.

4. Kopp AF, Mortele KJ, Cho YD, Palkowitsch P, Bettmann MA, Claussen CD. Prevalence of acute reactions to iopromide: postmarketing surveillance study of 74,717 patients. Acta Radiol. 2008. 49:902–911.

5. Palmer FJ. The RACR survey of intravenous contrast media reactions. Final report. Australas Radiol. 1988. 32:426–428.

6. ACR Committee on Drugs and Contrast Media. ACR manual on contrast media. Version 7. Table 3. Categories of reactions. 2010. 67. Available from: http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/Contrast%20Manual/ABCD%20Approach%20for%20Patient%20Evaluation%20and%20Treatment.pdf.
7. Australian Government. Australian weather and the seasons. Available from: http://www.cultureandrecreation.gov.au/articles/weather/.
8. Mortelé KJ, Oliva MR, Ondategui S, Ros PR, Silverman SG. Universal use of nonionic iodinated contrast medium for CT: evaluation of safety in a large urban teaching hospital. AJR Am J Roentgenol. 2005. 184:31–34.

9. Cochran ST, Bomyea K, Sayre JW. Trends in adverse events after IV administration of contrast media. AJR Am J Roentgenol. 2001. 176:1385–1388.

10. Caro JJ, Trindade E, McGregor M. The risks of death and of severe nonfatal reactions with high- vs low-osmolality contrast media: a meta-analysis. AJR Am J Roentgenol. 1991. 156:825–832.

11. Canter LM. Anaphylactoid reactions to radiocontrast media. Allergy Asthma Proc. 2005. 26:199–203.
12. Callahan MJ, Poznauskis L, Zurakowski D, Taylor GA. Nonionic iodinated intravenous contrast material-related reactions: incidence in large urban children's hospital--retrospective analysis of data in 12,494 patients. Radiology. 2009. 250:674–681.

13. Mikkonen R, Vehmas T, Granlund H, Kivisaari L. Seasonal variation in the occurrence of late adverse skin reactions to iodine-based contrast media. Acta Radiol. 2000. 41:390–393.

14. Brockow K, Romano A, Aberer W, Bircher AJ, Barbaud A, Bonadonna P, Faria E, Kanny G, Lerch M, Pichler WJ, Ring J, Rodrigues Cernadas J, Tomaz E, Demoly P, Christiansen C. European Network of Drug Allergy and the EAACI interest group on drug hypersensitivity. Skin testing in patients with hypersensitivity reactions to iodinated contrast media - a European multicenter study. Allergy. 2009. 64:234–241.

15. Laroche D, Aimone-Gastin I, Dubois F, Huet H, Gérard P, Vergnaud MC, Mouton-Faivre C, Guéant JL, Laxenaire MC, Bricard H. Mechanisms of severe, immediate reactions to iodinated contrast material. Radiology. 1998. 209:183–190.

16. Brockow K, Christiansen C, Kanny G, Clément O, Barbaud A, Bircher A, Dewachter P, Guéant JL, Rodriguez Guéant RM, Mouton-Faivre C, Ring J, Romano A, Sainte-Laudy J, Demoly P, Pichler WJ. ENDA. EAACI interest group on drug hypersensitivity. Management of hypersensitivity reactions to iodinated contrast media. Allergy. 2005. 60:150–158.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download