Abstract
Recent technical approaches to investigating drug hypersensitivity have provided a great deal of information to solve the mechanisms that remain poorly understood. First, immunological investigations and in silico analysis have revealed that a novel interaction between T cells and antigen-presenting cells, namely the pharmacological interaction concept, is involved in drug recognition and the hapten theory. Second, progress in immunology has provided a new concept of CD4+ T cell subsets. Th17 cells have proven to be a critical player in acute generalized exanthematous pustulosis. Our recent findings suggest that this subset might contribute to the pathogenesis of Stevens-Johnson syndrome/toxic epidermal necrolysis. Third, alarmins, molecules associated with innate immunity, are also associated with exaggeration and the persistence of severe drug hypersensitivity. The latest innovative techniques are providing a new landscape to examine drug hypersensitivity.
Why does drug eruption occur? Why does inflammation aggravate even if administration of the culprit drug is stopped? Unfortunately, we have no answers to these questions. However, the recent technical approach of the gene expression analysis has provided a great deal of information to solve them. Genome-wide analysis has revealed that individuals with certain human leukocyte antigen (HLA) polymorphisms have a 10 to more than 1,000-fold risk of drug hypersensitivity [1-4]. It will provide us with an order-made treatment to prevent severe drug hypersensitivity. Furthermore, recent in silico analysis makes it possible to reveal exquisite interactions between HLA molecules and drugs in the drug-antigen recognition of T cells [5], thereby providing us with a new perspective for drug discovery without hypersensitivity. In addition, recent studies using gene expression analysis have enabled the search for aggravation factors of severe drug hypersensitivity [6], which provides us with a novel therapeutic strategy. The latest innovative techniques are providing a new landscape to examine drug hypersensitivity.
Because most drugs are small molecules, their covalent binding to self-derived proteins, such as membrane proteins and serum albumin, is necessary for immunogenicity. Essentially, a new epitope is presented by covalent binding of drugs with self-proteins, which is recognized by T cells as a haptenic antigen. It has been accepted as an important event for small molecules to be immunogenic to elicit contact dermatitis in experimental models [7], and may be central to the mechanism of drug allergies (Fig. 1A) [8]. However, it has been recently demonstrated that a high affinity of the drug for major histocompatibility complex (MHC) expressed on antigen-presenting cells (APCs), or for T cell receptors (TCRs) expressed on T cells, increases the possibility of activating T cells (Figs. 1B and C) [8]. Such binding is non-covalent with electrical or van der Waals forces, and can be easily dissociated by mechanical force. This type of interaction between T cells and APCs in drug recognition has been proposed as the pharmacological interaction concept (p-i concept) by Pichler et al. [9]. Interestingly, in several previous reports, anticonvulsant-specific T cell clones tend to have Vβ 5.1, despite the diversity of HLA alleles [10-13]. Under such conditions, antigen processing appears unnecessary for T cell activation because T cells activate even in the presence of APCs fixed with glutaraldehyde and the culprit drug [12]. Some clones usually do not require HLA matching of APCs for the response to the drug, indicating non-MHC restricted T cell recognition [14]. Such observations are accounted for by the "p-i concept" binding between the drug and TCR. On the other hand, in Han Chinese, individuals with HLA-B*1502 have a more than 2,500-fold risk of carbamazepine hypersensitivity [4]. Recent computer analyses of the molecular structure revealed an exquisite interaction between two molecules in the drug-antigen recognition. In such cases, the drug non-covalently binds to HLA-B 15:02, which is indicative of the "p-i concept" style binding [5].
In the hapten theory, after sensitization with the haptenized drug antigen, memory T cells recognize the antigenic epitope presented by APCs, with the arms of the TCR to critically fit its structure. However, the unique drug recognition manner in the p-i concept does not require the sensitization of T cells to the drug [9]. Basically, memory T cells that express TCRs with a certain affinity to the drug activate even if they are not sensitized to the drug, in the presence of the drug and APCs. Japanese individuals with HLA-A*3101 have a greater risk of carbamazepine hypersensitivity [3] in addition to Han Chinese with HLA-B*1502 [4]. Such cases show no correlation between this HLA polymorphism and the severity of drug hypersensitivity, suggesting that other factor(s) may contribute to exacerbate drug hypersensitivity. The size of the TCR repertoire of memory T cells is influenced by the individual's previous infections. The larger the size of the TCR repertoire of memory T cells, the higher the possibility of drug affinity for TCRs. Therefore, the number of T cells that bind a drug in the manner of the "p-i concept" may be increased in individuals who have experienced more infections. This scenario is well fitted with our clinical observations, although further investigation is needed to address this issue.
Effector T cells are divided into CD4+ and CD8+ subtypes, and the former is classified into Th0, Th1, Th2, Th9, Th17 and Th22, depending on the profile of released cytokines (Fig. 2) [15]. The quality and quantity of activated T cells reflects the clinical skin manifestations of drug hypersensitivity. The severity of CD8+ T cell infiltration into the skin is correlated with the degree of epidermal cell necrosis, which determines the clinical phenotype such as maculopapular eruption, Stevens-Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN) (Fig. 3) [12]. In SJS/TEN, CD8+ T cells in blisters greatly activate to gain a natural killer (NK) cell marker, CD94/NKG2c, on their cell surface and acquire an antigen-independent killing activity resembling that of NK cells via binding to classical MHC (HLA-E) expressed on inflammatory epidermal cells [16]. Therefore, the activation status of CD8+ T cells in skin and blood is a predisposing factor for severe drug hypersensitivity such as SJS/TEN. On the other hand, a Th2-shifted immune response induces tissue eosinophilia and overproduction of IgE, resulting in the emergence of edematous erythema and wheals. β-lactam-specific T cells tend to produce Th2 cytokines such as interleukin (IL)-4, IL-5 and IL-13, which is indicative of Th2 cells and consistent with the clinical presentations [17]. IL-17-producing T cells, known as Th17 cells, are attracting attention as pathognomonic T cells in psoriasis, which also produce IL-8 (CXCL-8), a chemokine for neutrophils [18]. High serum levels of IL-17 and IL-8 are observed in acute generalized exanthematous pustulosis that clinically resembles pustular psoriasis, suggesting a contribution of Th17 to the pathology [19, 20]. Th17 cells cooperatively exaggerate the inflammatory responses of Th1/Tc1 [18]. Interestingly, CD4+ T cells with the ability to produce high levels of IL-17 are found among the drug-specific CD4+ T cell clones established from SJS/TEN patients (Fig. 4, unpublished data). Damaged epidermal cells in SJS/TEN release prostaglandin E2 and alarmins, which promotes differentiation and proliferation of Th17 cells activated by IL-23-producing dendritic cells after signaling via their receptors. This observation may be one of the explanations for the persistence of these serious diseases.
Regulatory T cells (Tregs) are another modifier of the inflammatory responses in drug hypersensitivity, which comprise natural and induced CD4+CD25+Foxp3+ Tregs and other cell types. Because of functional impairment of CD4+CD25+Foxp3+ Tregs [21-26], the risk of drug hypersensitivity would tend to rise in autoimmune disorders [27]. Anti-CCR4 antibody treatment is a novel therapy for adult T cell leukemia lymphoma (ATL/L) to kill CCR4-expressing ATL/L cells, while it may also reduce the number of CD4+CD25+Foxp+ Tregs that express CCR4. During such treatment, drug eruptions including SJS/TEN develop in around 70% of cases, suggesting that impairment of Tregs is a high risk factor for drug hypersensitivity. Functional impairment of Tregs has been found in drug-induced hypersensitivity syndrome (DIHS), which may be associated with a prolonged disease course [27].
Oppenheim proposed that damage associated molecular pattern molecules (DAMPs) released from damaged cells are cues for initiating immune responses in various organs by their activation after interacting with pattern recognition receptors and/or toll-like receptors [28, 29]. These molecules promote rapid recruitment of bone marrow-derived leukocytes to target tissues for inflammation and regeneration under various aseptic inflammatory conditions including SJS and TEN [6]. This molecular group contains various substances from simple chemicals, such as uric acid, to larger molecules such as IL-33 and DNA. Endogenous DAMPs are now designated as alarmins. High mobility group box-1 (HMGB-1), one of the most well-known alarmin members, is a non-histone protein with dual functions, namely transcriptional regulation by loose binding to chromatin inside cells and a cue for inflammation outside cells, with high potency to attract and activate various immunocompetent cells including monocytes and myeloid cells [28]. Recently, high expression levels of HMGB-1 have been found in blood from SJS patients [30]. Granulysin is a member of the saposin-like protein family, which is classified into 9- and 15-kDa isoforms. The 9-kDa granulysin is a well-described pore forming cytotoxic molecule with proinflammatory activity, and is considered as a crucial molecule to induce apoptosis of keratinocytes in SJS/TEN [31]. The 15-kDa granulysin has been recently accepted as an alarmin, which activates monocytes and dendritic cells via binding the toll-like receptor-4/Myd88, rather than a cytotoxic molecule [32, 33]. In addition, 15-kDa granulysin may act as an alarmin in SJS/TEN to promote Th17 cell responses by activation of dendritic cells.
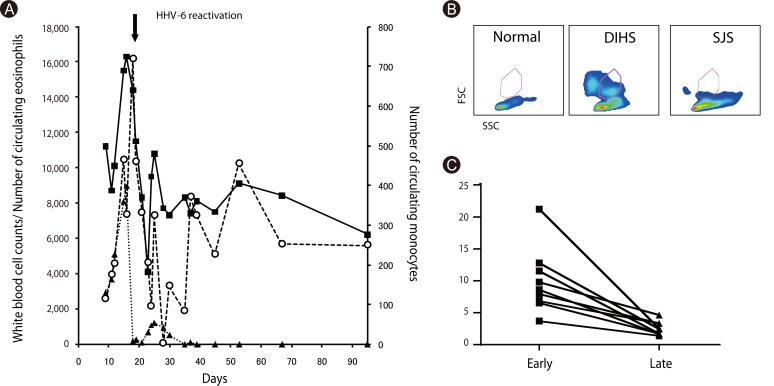
The most mysterious phenomenon in DIHS is reactivation of various HHV types, including cytomegalovirus, Epstein-Barr virus, HHV-6 and HHV-7, during the disease course [34, 35]. HHV-6 reactivation has been found in more than 60% of such cases in association with unfavorable outcomes [36]. Our concern is why HHV-6 frequently reactivates in DIHS. We found that the number of circulating monocytes transiently increase (>500/mm3) within 3 weeks and return to normal levels thereafter (Figs. 5A and C). The monocytes transiently observed in DIHS are different from conventional monocytes in terms of side scatter counts (SSC) and phenotype [37]. Monocytes from DIHS patients show higher SCC (Fig. 5B), suggesting enriched organelles, and comprise a minor CD14highCD16- population and a major CD14lowCD16+ population that express skin-associated molecules such as CCR4, CLA markedly and CCR10 partially (manuscript in preparation). This observation implies that they are a precursor mono/myeloid subset that differentiates into skin-resident macrophages. We also found the HHV-6 genome and virus structures in some of these monocytes (manuscript in preparation). Because some monomyeloid cells are latently infected with HHV-6 as virus reservoirs [38], monocytes that transiently circulate in DIHS patients appear to have originated from such cells. We found close contacts between monocytes and T cells in the skin, and the presence of HHV-6 in skin-resident CD4+ T cells (manuscript in preparation). HHV-6 infection of CD4+ T cells is an indispensable event for virus replication and reactivation [39]. Monocytes may lead this critical event in CD4+ T cells, suggesting that HHV-6 infects CD4+ T cells in the skin of DIHS patients. This notion provides new perspectives for understanding the pathology of DIHS to correlate allergy with viral infection.
The recent technical approach of gene expression analysis has provided a great deal of information to reveal the mechanisms of drug hypersensitivity. Consequently, a new approach with in silico analysis is being developed for clarification of the exquisite interaction between drugs and HLAs or TCRs. The Ministry of Health, Labour and Welfare in Japan recently reported that there were 131 deaths over 2.5 years owing to drug hypersensitivity. To overcome this iatrogenic death, we should make an effort to clarify the mechanisms and to establish an effective treatment. We believe that these advances may avoid drug hypersensitivity reactions in the near future.
References
1. Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, Lin YL, Lan JL, Yang LC, Hong HS, Chen MJ, Lai PC, Wu MS, Chu CY, Wang KH, Chen CH, Fann CS, Wu JY, Chen YT. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005; 102:4134–4139. PMID: 15743917.

2. Martin AM, Nolan D, Gaudieri S, Almeida CA, Nolan R, James I, Carvalho F, Phillips E, Christiansen FT, Purcell AW, McCluskey J, Mallal S. Predisposition to abacavir hypersensitivity conferred by HLA-B*5701 and a haplotypic Hsp70-Hom variant. Proc Natl Acad Sci U S A. 2004; 101:4180–4185. PMID: 15024131.

3. Ozeki T, Mushiroda T, Yowang A, Takahashi A, Kubo M, Shirakata Y, Ikezawa Z, Iijima M, Shiohara T, Hashimoto K, Kamatani N, Nakamura Y. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum Mol Genet. 2011; 20:1034–1041. PMID: 21149285.

4. Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, Wu JY, Chen YT. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004; 428:486. PMID: 15057820.
5. Wei CY, Chung WH, Huang HW, Chen YT, Hung SI. Direct interaction between HLA-B and carbamazepine activates T cells in patients with Stevens-Johnson syndrome. J Allergy Clin Immunol. 2012; 129:1562–1569.e5. PMID: 22322005.

6. Bellón T, Alvarez L, Mayorga C, Morel E, Torres MJ, Martín-Díaz MA, Díaz R, Radial A, Carballo M, Blanca M. Differential gene expression in drug hypersensitivity reactions: induction of alarmins in severe bullous diseases. Br J Dermatol. 2010; 162:1014–1022. PMID: 20030638.

7. Jenkinson C, Jenkins RE, Aleksic M, Pirmohamed M, Naisbitt DJ, Park BK. Characterization of p-phenylenediamine-albumin binding sites and T-cell responses to hapten-modified protein. J Invest Dermatol. 2010; 130:732–742. PMID: 19710686.

8. Adam J, Pichler WJ, Yerly D. Delayed drug hypersensitivity: models of T-cell stimulation. Br J Clin Pharmacol. 2011; 71:701–707. PMID: 21480949.

9. Pichler WJ, Beeler A, Keller M, Lerch M, Posadas S, Schmid D, Spanou Z, Zawodniak A, Gerber B. Pharmacological interaction of drugs with immune receptors: the p-i concept. Allergol Int. 2006; 55:17–25. PMID: 17075282.

10. Naisbitt DJ, Farrell J, Wong G, Depta JP, Dodd CC, Hopkins JE, Gibney CA, Chadwick DW, Pichler WJ, Pirmohamed M, Park BK. Characterization of drug-specific T cells in lamotrigine hypersensitivity. J Allergy Clin Immunol. 2003; 111:1393–1403. PMID: 12789244.

11. Naisbitt DJ, Britschgi M, Wong G, Farrell J, Depta JP, Chadwick DW, Pichler WJ, Pirmohamed M, Park BK. Hypersensitivity reactions to carbamazepine: characterization of the specificity, phenotype, and cytokine profile of drug-specific T cell clones. Mol Pharmacol. 2003; 63:732–741. PMID: 12606784.

12. Hashizume H, Takigawa M, Tokura Y. Characterization of drug-specific T cells in phenobarbital-induced eruption. J Immunol. 2002; 168:5359–5368. PMID: 11994495.
13. Krivoy N, Taer M, Neuman MG. Antiepileptic drug-induced hypersensitivity syndrome reactions. Curr Drug Saf. 2006; 1:289–299. PMID: 18690940.

14. Hashizume H, Seo N, Ito T, Takigawa M, Yagi H. Promiscuous interaction between gold-specific T cells and APCs in gold allergy. J Immunol. 2008; 181:8096–8102. PMID: 19018002.

15. Annunziato F, Romagnani S. Heterogeneity of human effector CD4+ T cells. Arthritis Res Ther. 2009; 11:257. PMID: 20053303.

16. Morel E, Escamochero S, Cabañas R, Díaz R, Fiandor A, Bellón T. CD94/NKG2C is a killer effector molecule in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Allergy Clin Immunol. 2010; 125:703–710. 710.e1–710.e8. PMID: 20132973.

17. Brugnolo F, Annunziato F, Sampognaro S, Campi P, Manfredi M, Matucci A, Blanca M, Romagnani S, Maggi E, Parronchi P. Highly Th2-skewed cytokine profile of beta-lactam-specific T cells from nonatopic subjects with adverse drug reactions. J Immunol. 1999; 163:1053–1059. PMID: 10395704.
19. Schaerli P, Britschgi M, Keller M, Steiner UC, Steinmann LS, Moser B, Pichler WJ. Characterization of human T cells that regulate neutrophilic skin inflammation. J Immunol. 2004; 173:2151–2158. PMID: 15265952.

20. Britschgi M, Steiner UC, Schmid S, Depta JP, Senti G, Bircher A, Burkhart C, Yawalkar N, Pichler WJ. T-cell involvement in drug-induced acute generalized exanthematous pustulosis. J Clin Invest. 2001; 107:1433–1441. PMID: 11390425.

21. Matsumoto Y, Sakuma H, Kohyama K, Park IK. Paralysis of CD4(+) CD25(+) regulatory T cell response in chronic autoimmune encephalomyelitis. J Neuroimmunol. 2007; 187:44–54. PMID: 17499858.
22. Kelchtermans H, De Klerck B, Mitera T, Van Balen M, Bullens D, Billiau A, Leclercq G, Matthys P. Defective CD4+CD25+ regulatory T cell functioning in collagen-induced arthritis: an important factor in pathogenesis, counter-regulated by endogenous IFN-gamma. Arthritis Res Ther. 2005; 7:R402–R415. PMID: 15743488.
23. Balandina A, Lécart S, Dartevelle P, Saoudi A, Berrih-Aknin S. Functional defect of regulatory CD4(+)CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood. 2005; 105:735–741. PMID: 15454488.

24. Nishibori T, Tanabe Y, Su L, David M. Impaired development of CD4+ CD25+ regulatory T cells in the absence of STAT1: increased susceptibility to autoimmune disease. J Exp Med. 2004; 199:25–34. PMID: 14699080.
25. Longhi MS, Ma Y, Bogdanos DP, Cheeseman P, Mieli-Vergani G, Vergani D. Impairment of CD4(+)CD25(+) regulatory T-cells in autoimmune liver disease. J Hepatol. 2004; 41:31–37. PMID: 15246204.

26. Mao C, Wang S, Xiao Y, Xu J, Jiang Q, Jin M, Jiang X, Guo H, Ning G, Zhang Y. Impairment of regulatory capacity of CD4+CD25+ regulatory T cells mediated by dendritic cell polarization and hyperthyroidism in Graves' disease. J Immunol. 2011; 186:4734–4743. PMID: 21398613.

27. Takahashi R, Kano Y, Yamazaki Y, Kimishima M, Mizukawa Y, Shiohara T. Defective regulatory T cells in patients with severe drug eruptions: timing of the dysfunction is associated with the pathological phenotype and outcome. J Immunol. 2009; 182:8071–8079. PMID: 19494333.

28. Oppenheim JJ, Tewary P, de la Rosa G, Yang D. Alarmins initiate host defense. Adv Exp Med Biol. 2007; 601:185–194. PMID: 17713005.

29. Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010; 2010:672395. PMID: 20706656.

30. Nakajima S, Watanabe H, Tohyama M, Sugita K, Iijima M, Hashimoto K, Tokura Y, Nishimura Y, Doi H, Tanioka M, Miyachi Y, Kabashima K. High-mobility group box 1 protein (HMGB1) as a novel diagnostic tool for toxic epidermal necrolysis and Stevens-Johnson syndrome. Arch Dermatol. 2011; 147:1110–1112. PMID: 21931056.

31. Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, Chin SW, Chiou CC, Chu SC, Ho HC, Yang CH, Lu CF, Wu JY, Liao YD, Chen YT. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008; 14:1343–1350. PMID: 19029983.

32. Clayberger C, Finn MW, Wang T, Saini R, Wilson C, Barr VA, Sabatino M, Castiello L, Stroncek D, Krensky AM. 15 kDa granulysin causes differentiation of monocytes to dendritic cells but lacks cytotoxic activity. J Immunol. 2012; 188:6119–6126. PMID: 22586033.

33. Tewary P, Yang D, de la Rosa G, Li Y, Finn MW, Krensky AM, Clayberger C, Oppenheim JJ. Granulysin activates antigen-presenting cells through TLR4 and acts as an immune alarmin. Blood. 2010; 116:3465–3474. PMID: 20660289.

34. Seishima M, Yamanaka S, Fujisawa T, Tohyama M, Hashimoto K. Reactivation of human herpesvirus (HHV) family members other than HHV-6 in drug-induced hypersensitivity syndrome. Br J Dermatol. 2006; 155:344–349. PMID: 16882173.

35. Kano Y, Hiraharas K, Sakuma K, Shiohara T. Several herpesviruses can reactivate in a severe drug-induced multiorgan reaction in the same sequential order as in graft-versus-host disease. Br J Dermatol. 2006; 155:301–306. PMID: 16882166.

36. Tohyama M, Hashimoto K, Yasukawa M, Kimura H, Horikawa T, Nakajima K, Urano Y, Matsumoto K, Iijima M, Shear NH. Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br J Dermatol. 2007; 157:934–940. PMID: 17854362.

37. Hashizume H, Aoshima M, Ito T, Seo N, Takigawa M, Yagi H. Emergence of circulating monomyeloid precursors predicts reactivation of human herpesvirus-6 in drug-induced hypersensitivity syndrome. Br J Dermatol. 2009; 161:486–488. PMID: 19485994.

38. Kondo K, Kondo T, Okuno T, Takahashi M, Yamanishi K. Latent human herpesvirus 6 infection of human monocytes/macrophages. J Gen Virol. 1991; 72:1401–1408. PMID: 1646280.

39. Dockrell DH. Human herpesvirus 6: molecular biology and clinical features. J Med Microbiol. 2003; 52:5–18. PMID: 12488560.

Fig. 1
Interaction of T cells and antigen-presenting cells (APCs) in drug antigen recognition. A new epitope is presented by covalent binding of drugs with self-proteins, which is recognized by T cells as a haptenic antigen (A). On the other hand, a high affinity of the drug for T cell receptors (TCRs) expressed on T cells (B), or major histocompatibility complex (MHC) expressed on APCs (C), increases the possibility of activating T cells (p-i concept, modified in the Fig. of ref. [9]).
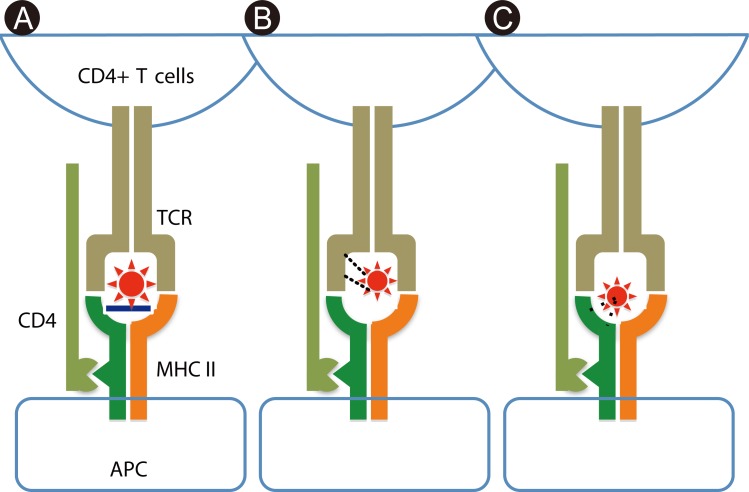
Fig. 2
Functional subsets of effector CD4+ T cells [15]. APC, antigen-presenting cell; GATA3, the transcription factor GATA-binding protein-3; IFN, interferon; TGF, transforming growth factor; AHR, aryl hydrocarbon receptor; RORγt, retinoic acid-related orphan receptor γt; T-bet, T-box expressed in T cells; DTH, delayed type hypersensitivity; EAE, experimental autoimmune encephalomyelitis; EAU, experimental autoimmune uveitis; CIA, collagen-induced arthritis; PIA, peptoglycan-induced arthritis.
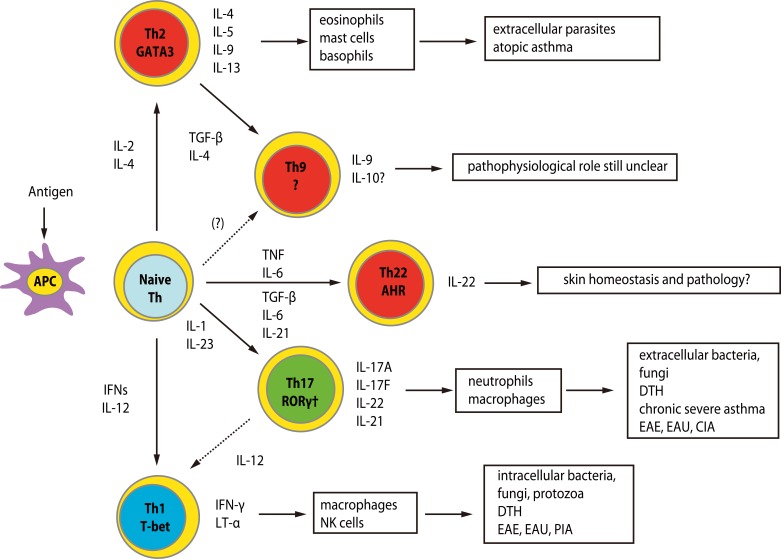
Fig. 3
Phenotypic analysis of peripheral blood mononuclear cells (PBMCs) and mononuclear cells in blisters (Blisters) in a toxic epidermal necrolysis patient. Numbers indicate percentages of total cells.
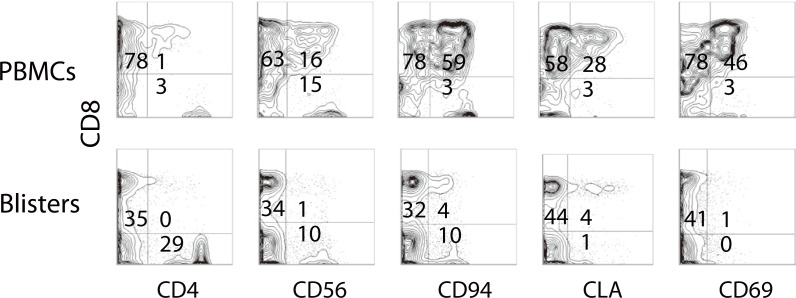
Fig. 4
IFN-γ and IL-17 productions of drug-specific T cell clones (TCCs) after stimulation with anti-CD3 antibody for 3 days. TCCs were established from patients with gold and nickel allergies (Metal), SJS/TEN and DIHS. Y-axis, cytokine concentrations (pg/mL). *p < 0.05, Dunn's Multiple Comparison Test. SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis; DIHS, drug-induced hypersensitivity syndrome.
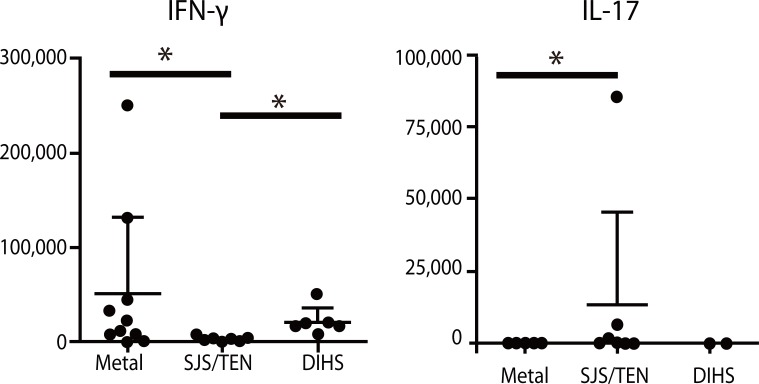
Fig. 5
Circulating monocytes in drug-induced hypersensitivity syndrome (DIHS) patients. (A) Representative data of total blood cell counts (closed squares), numbers of eosinophils (closed triangles) and monocytes (open circles). Numbers indicate cell counts (/mm3). An arrow indicates human herpes virus (HHV)-6 reactivation. (B) Flowcytometric analysis of peripheral blood mononuclear cells in normal individuals and patients with DIHS and Stevens-Johnson syndrome (SJS). Monocytes from DIHS patients show higher side scatter counts (SCC). FSC, forward scatter counts. (C) Percentage of monocytes at early (≤21 days after the onset) and late (>21 days after the onset) phase.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download