This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Olopatadine hydrochloride ophthalmic solutions are treated for allergic conjunctival diseases that are a selective histamine H1 receptor antagonist and an inhibitor of the release of mediators including histamine from the human mast cells. Substance P (SP) levels are increased in tears of patients with allergic conjunctivitis. However, little is known about the regulation of SP release by anti-allergic ophthalmic solutions.
Objective
We investigated that the effect of olopatadine hydrochloride ophthalmic solutions (olopatadine 0.1% and olopatadine 0.2%) on rat conjunctivitis models compared with other anti-allergic ophthalmic solutions.
Methods
Conjunctivitis was induced by subconjunctival injection of histamine or intravenous injection of ovalbumin in rats passively sensitized with anti-ovalbumin anti-serum. The releases of SP were determined in the conjunctiva and tears using rat antigen-induced conjunctivitis models.
Results
Olopatadine 0.1% and 0.2% significantly inhibited the increased conjunctival dye leaked in the histamine- or antigen-induced hyperpermeability. The inhibitory effects by olopatadine were more potent than by other tested anti-allergic ophthalmic solutions. Moreover, olopatadine significantly inhibited the release of SP from the conjunctiva.
Conclusion
These results indicate that olopatadine ophthalmic solutions appear to exert additional SP release inhibition besides dual-action such as selective histamine H1 receptor antagonistic action and mast cell stabilization action.
Go to :

Keywords: Olopatadine hydrochloride, Ophthalmic solution, Substance P, Allergic conjunctivitis, Rat
INTRODUCTION
Allergic diseases such as seasonal or perennial allergic conjunctivitis (AC) are closely related to a type I allergic reaction triggered by the allergen on the conjunctival mast cell. AC is caused by an allergen-induced inflammatory response, in which allergen-specific IgE antibodies bind to mast cells in the conjunctiva, triggering the release of chemical mediators, including histamine and cytokines. Histamine binding to its receptors plays a central role in the induction of major allergic symptoms such as itching.
Choices for the treatment of AC are anti-allergy ophthalmic solution, steroid ophthalmic solution, and immunosuppressant ophthalmic solution; the first choice for the subject to the immediate phase of allergic inflammation may be anti-allergic ophthalmic solution.
Olopatadine hydrochloride ophthalmic solution (olopatadine) inhibits the binding of histamine to histamine H1 receptor, with superior histamine H1 receptor selectivity [
1-
3]. Olopatadine has also been shown to stabilize human conjunctival mast cells [
1,
3,
4].
It has been reported that substance P (SP) levels are increased in tears of patients with AC compared with healthy individuals, suggesting that SP may contribute to the pathogenesis and severity of AC [
5-
7]. There are a variety of known itch-associated mediators in dermatitis, including histamine, neuropeptides (SP, calcitonin gene-related peptide, etc.), opioids, growth factors, cytokines, etc [
8,
9]. Oral olopatadine was reported to mitigate cutaneous inflammation and the increased number of scratching episodes in a mouse model of chronic inflammatory dermatitis, accompanied by decreased cytokine and SP productions [
10,
11]. However, little is known about the regulation of SP release by anti-allergic ophthalmic drugs in AC.
The aim of this study is to supplement the known pharmacological profile of olopatadine with the commonly used anti-allergic ophthalmic solutions, levocabastine and tranilast in experimental rat conjunctivitis models. In addition, the release of SP was determined in the conjunctiva and tears using rat antigen-induced conjunctivitis models.
Go to :

MATERIALS AND METHODS
Rats
Male 6-week-old Wistar rats were purchased from Charles River Japan (Japan). Animals were maintained at 19-25℃ with 30-70% of humidity on a 12 h light/dark cycle (light on at 7:00), and had free access to commercial pellets and water. Experiments were performed in accordance with recommendations of the institutional animal care and use committee of the Kyowa Hakko Kirin Co., Ltd. (Japan).
Reagents
Ophthalmic solutions used in this study were olopatadine hydrochloride ophthalmic solutions (Patanol® ophthalmic solution 0.1%, olopatadine 0.1%; Kyowa Hakko Kirin, Japan and Pataday™ ophthalmic solution 0.2%, olopatadine 0.2%; Alcon Laboratories, USA), levocabastine ophthalmic solution (Livostin® Eye Drops 0.025%, levocabastine; Janssen Pharmaceutical, Japan), and tranilast ophthalmic solution (Rizaben® Eye Drops 0.5%, tranilast; Kissei Pharmaceutical, Japan). Reagents used in this study were histamine dihydrochloride (Histamine; Sigma-Aldrich, USA), albumin (Ovalbumin, OVA; Sigma-Aldrich, USA) and Evans blue (Sigma-Aldrich, USA).
Histamine-induced conjunctival vascular hyperpermeability in rats
To compare the anti-histamine efficacy, conjunctival vascular hyperpermeability was induced by histamine in rats. Rats were injected with 30 µL of histamine (0.1 mg/mL) by the upper subconjunctiva under isoflurane anesthesia immediately after the intravenous injection of 1.5 w/v% Evans blue solution. Thirty min later, the animals were sacrificed and the bluing eye (eyelids and eyeballs) was removed. Dye which had leaked into the tissue was extracted with formamide at 45℃ and its absorbance at 625 nm was determined with a spectrophotometer (THERMOmax™; Molecular Devices, USA). Values were analyzed as per weight of each organization. Ophthalmic solutions or saline were applied to the eyes (10 µL per eye) 30 min prior to the challenge of histamine. As negative control, rats were injected with 30 µL of saline by the upper subconjunctiva and were injected with 1.5 w/v% Evans blue solution.
Antigen-induced conjunctival vascular hyperpermeability in passively sensitized rats
Conjunctival vascular hyperpermeability were induced by an experimental AC in passively sensitized rats. Anti-OVA antiserum containing IgE antibody was prepared by the method of Stotland and Share [
12] using male rats. Rats were passively sensitized with 30 µL of anti-OVA antiserum by the upper subconjunctival injection under isoflurane anesthesia. Forty-eight h after the sensitization, the animals were subjected to challenge by an intravenous injection of OVA (2 mg/mL) solution containing 1.5 w/v% Evans blue. Thirty min later, the animals were sacrificed and the bluing eye (eyelids and eyeballs) was removed. Dye which had leaked into the tissue was determined by the method described above. Ophthalmic solutions or saline were applied to the eyes (10 µL per eye) 30 min prior to the challenge of antigen. As negative control, sensitized rats were injected with 1.5 w/v% Evans blue.
The onset and duration of action of olopatadine on the conjunctival vascular hyperpermeability in antigen-induced conjunctival vascular hyperpermeability
Conjunctival vascular hyperpermeability were induced by an experimental AC in passively sensitized rats. Ophthalmic solutions or saline were applied to the eyes (10 µL per eye) 24 h, 6 h prior to the challenge of antigen, or 5 min after the challenge of antigen. As negative control, sensitized rats were injected with 1.5 w/v% Evans blue.
Measurement of SP in conjunctiva
We determined the levels of SP in the conjunctiva using rat antigen-induced conjunctivitis models. Sensitized rats were subjected to challenge by an intravenous injection of OVA solution. Ophthalmic solutions were instilled at 30 min before antigen challenge and immediately after antigen challenge into the bilateral eye with 10 µL/site. Thirty min later, the animals were sacrificed and the conjunctivas were removed. The removed tissue was homogenized in phosphate buffered saline containing the protease inhibitor. After centrifugation, the supernatant was collected and stored at -35℃. SP levels were determined using commercial SP assay kit (Cayman Chemical, USA) according to the provided protocol. The absorbance was measured using a microplate spectrophotometer. The determined values were analyzed as values per total protein. The percentage of SP release calculated [
13]: SP release (%) = [(Average of SP level of the conjunctiva in the pre group) - (Average of SP level of the conjunctiva in the control group or the drug treated group)] / (Average of SP level of the conjunctiva in the pre group) × 100.
Measurement of SP in tears
Moreover, we determined the levels of SP in tears using rat antigen-induced conjunctivitis models. Another sensitized rat was subjected to challenge by an intravenous injection of OVA solution. Thirty min later, 50 µL saline was applied to both eyes. This procedure was repeated 3 times and a 200 µL sample was carefully collected from both eyes. After centrifugation, the supernatant was collected and SP levels were determined using commercial SP assay kit according to the provided protocol. The determined values were analyzed as values per total protein.
Statistical analysis
Data were presented as means ± SE. The Aspin-Welch test or Student's t-test following the F-test was used for analysis of differences between two groups. Values of p < 0.05 were considered statistically significant.
Go to :

RESULTS
Histamine-induced conjunctival vascular hyperpermeability in rats
The amount of dye leaked by the injection of histamine into the upper subconjunctiva was significantly increased compared to saline-injected animals (
p < 0.0001) (
Fig. 1A). Olopatadine 0.1% and olopatadine 0.2% significantly suppressed the increased conjunctival dye leaked (
p < 0.0001,
p < 0.0001). Levocabastine also significantly inhibited the increased conjunctival dye leaked by histamine (
p = 0.0007). Tranilast did not significantly inhibit. These inhibitory effects of olopatadine were more potent than for other tested anti-allergic ophthalmic solutions.
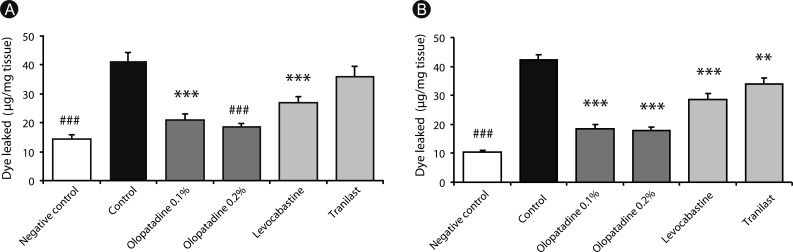 | Fig. 1Effects of olopatadine ophthalmic solution 0.1% and 0.2% compared other anti-allergic ophthalmic solutions on histamine (A) or ovalbumin (B)-induced conjunctival vascular hyperpermeability in passively sensitized rats. Olopatadine 0.1%, olopatadine 0.2%, levocabastine 0.025%, and tranilast 0.5% were applied topically onto the eye 30 min before the challenge of antigen and histamine with Evans blue dye. Each column and vertical bar represents the mean ± SE of 8-11 rats. **p < 0.01, ***p < 0.001: significantly different from the control group (Student's t-test). ###p < 0.001: significantly different from the control group (Aspin Welch test). 
|
Antigen-induced conjunctival vascular hyperpermeability in passively sensitized rats
To elucidate the ocular anti-allergic efficacy of olopatadine and other anti-allergic ophthalmic solutions, we investigated in rat antigen-induced conjunctivitis models (
Fig. 1B). The amount of dye leaked by the intravenous injection of OVA was significantly increased compared to saline-injected animals (
p < 0.0001). When olopatadine 0.1% and 0.2% were instilled into rats eyes 30 min before antigen challenge, the increased conjunctival dye leaked were significantly suppressed at both concentrations (
p < 0.0001,
p < 0.0001). Levocabastine and tranilast also significantly inhibited the increased conjunctival dye leaked by antigen (
p = 0.0001,
p = 0.0061). These inhibitory effects of olopatadine were more potent than for other tested anti-allergic ophthalmic solutions.
The onset and duration of action of olopatadine on antigen-induced conjunctival vascular hyperpermeability in passively sensitized rats
Next, the onset and duration of action of olopatadine on the conjunctival vascular hyperpermeability were investigated in rat AC (
Fig. 2). Ophthalmic solutions were applied topically onto eyes 24 h or 6 h prior to the challenge of antigen, or 5 min after the challenge of antigen. Pre-treatment 6 h with olopatadine 0.1% and 0.2% significantly inhibited the increased conjunctival dye leaked by 75.7% and 89.2% inhibition (
p < 0.0001,
p < 0.0001). Pre-treatment 24 h with olopatadine 0.1% and 0.2% significantly inhibited the increased conjunctival dye leaked by antigen by 71.6% and 80.4% inhibition (
p = 0.0001,
p = 0.0003). Moreover, when ophthalmic solutions were applied to the eyes 5 min after the challenge of antigen, olopatadine 0.1% and 0.2% also significantly inhibited the increased conjunctival dye leaked by 59.7% and 77.8% (
p = 0.0006,
p = 0.0002).
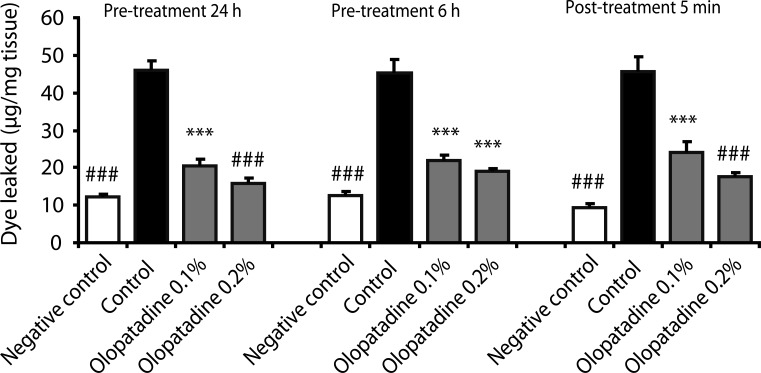 | Fig. 2Effects of olopatadine ophthalmic solution 0.1% and 0.2% on ovalbumin-induced conjunctival vascular hyperpermeability in passively sensitized rats. Olopatadine ophthalmic solution 0.1% (olopatadine 0.1%), olopatadine ophthalmic solution 0.2% (olopatadine 0.2%) were applied topically onto the eyes 24 h, 6 h prior to the challenge of antigen, and 5 min after the challenge of antigen. Each column and vertical bar represents the mean ± SE of 4-7 rats. ***p < 0.001: significantly different from the control group (Student's t-test). ###p < 0.001: significantly different from the control group (Aspin Welch test). 
|
Effect of olopatadine on SP release from the conjunctiva
Fig. 3A shows the effect of olopatadine and other anti-allergic ophthalmic solutions on SP release from the rat conjunctiva. SP contents in the conjunctiva was increased significantly by the OVA sensitization compared with normal rats (
p = 0.0088), while it was decreased significantly after the OVA challenge (
p = 0.0044), and the percentage of SP release by the OVA challenge was 33.1% (
Fig. 3B). Olopatadine 0.1% and 0.2% significantly inhibited the decreased conjunctival SP contents (
p = 0.0324,
p = 0.0040), and their percentages of SP release were 13.7% and 8.2%. Therefore, olopatadine inhibited SP release from the conjunctiva. On the other hand, other anti-allergic ophthalmic solutions did not inhibit the decrease in the levels of SP.
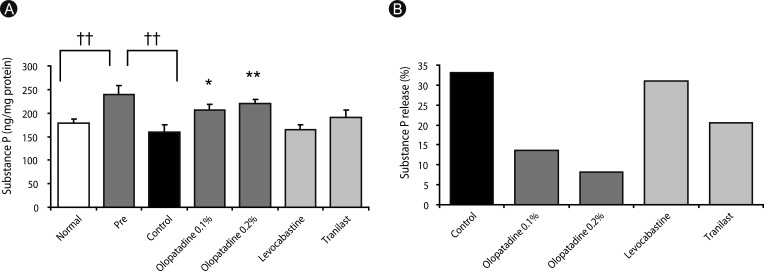 | Fig. 3Effects of olopatadine ophthalmic solution compared other anti-allergic ophthalmic solution on substance P (SP) contents (A) and SP release (B) in conjunctiva induced by ovalbumin in passively sensitized rats. SP contents were measured 30 min after antigen challenge. Ophthalmic solutions were instilled at 30 min before antigen challenge and immediately after antigen challenge into the bilateral eye with 10 µL/site. Each column and vertical bar of SP contents (A) represents the mean ± SE of 7-9 rats. *p < 0.05, **p < 0.01: significantly different from the control group (Student's t-test). ††p < 0.01: significantly different from the pre group (Student's t-test). 
|
Effect of olopatadine on the levels of SP in tears
To elucidate the SP release of olopatadine and other anti-allergic ophthalmic solutions, we determined the levels of SP in tears using rat antigen-induced conjunctivitis model (
Fig. 4). The levels of SP in tears were significantly increased by the OVA challenge compared with normal rats. Olopatadine 0.1% and 0.2% significantly inhibited the increase in the levels of SP in tears (
p = 0.0054,
p = 0.0005). The levels of SP in tears after application of olopatadine 0.2% was significantly less than those of application of olopatadine 0.1% (
p = 0.0225). Tranilast also significantly inhibited the increase in the levels of SP (
p = 0.0392). On the other hand, levocabastine did not inhibit the increase in the levels of SP.
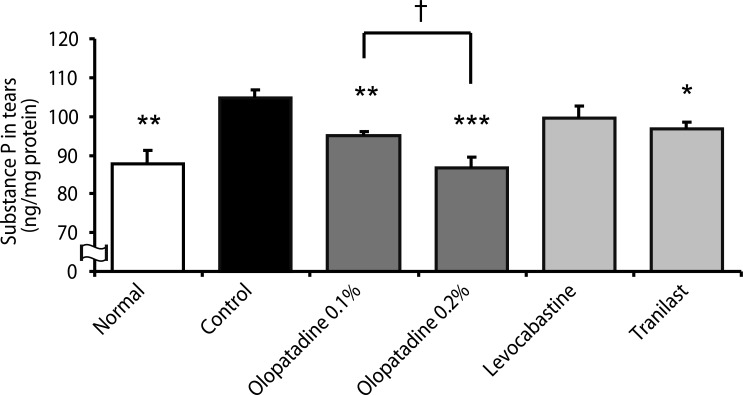 | Fig. 4Effects of olopatadine ophthalmic solution and other anti-allergic ophthalmic solutions on substance P (SP) contents in tears induced by ovalbumin in passively sensitized rats. SP contents were measured 30 min after antigen challenge. Ophthalmic solutions were instilled at 30 min before antigen challenge and immediately after antigen challenge into the bilateral eye at 10 µL/site. Each column and vertical bar represents the mean ± SE of 5-7 rats. *p < 0.05, **p < 0.01, ***p < 0.001: significantly different from the control group (Student's t-test). †p < 0.05: significantly different from the olopatadine 0.1% group (Student's t-test). 
|
Go to :

DISCUSSION
In this study, a rat model of conjunctivitis was used to examine the ocular anti-histamine and anti-allergic efficacies of olopatadine ophthalmic solution compared with other anti-allergic ophthalmic solutions. The present study also demonstrated that the SP levels were decreased in the conjunctiva and increased in tears in rat antigen-induced conjunctivitis models. This is in agreement with the clinical studies [
5,
14], which showed that the level of SP in tears was abundant at the onset of allergy symptoms.
The different mechanisms of action of the anti-allergy ophthalmic solutions possibly account for the observed differences in efficacy. Olopatadine ophthalmic solution is a highly effective dual-action drug with selective histamine H1 receptor antagonistic action and human conjunctival mast cell stabilization action. Levocabastine is a histamine H1 receptor antagonist. Tranilast is characterized as a mediator release inhibitor. At first, to compare the anti-histamine efficacy, conjunctival vascular hyperpermeability was induced by subconjunctival injection of histamine. It has demonstrated that olopatadine ophthalmic solution inhibits binding of histamine to H1 receptors, with superior H1 receptor selectivity compared with other anti-allergic ophthalmic solutions [
1-
3]. In this study, olopatadine 0.1% and 0.2% significantly inhibited the increased conjunctival dye leaked in the histamine-induced hyperpermeability model. These anti-histamine effects were more potent than for other tested anti-allergic ophthalmic solutions. In contrast, tranilast did not significantly inhibit.
Next, to compare the anti-allergic efficacy, antigen-induced conjunctivitis model was induced by intravenous injection of OVA in rats passively sensitized with anti-OVA antiserum. The inhibitory effects of olopatadine ophthalmic solutions in rat antigen-induced conjunctivitis models also were more potent than for other tested anti-allergic ophthalmic solutions. In this model, tranilast also significantly inhibit it.
Histamine and various cytokines are released from conjunctival epithelial cells and mast cells by antigen-antibody reaction [
1-
3]. Olopatadine ophthalmic solution has been shown to suppress the histamine release in a concentration-dependent manner from human conjunctival mast cells [
4,
15]. These effects of olopatadine 0.1% and 0.2% are continued and faster than other tested anti-allergic ophthalmic solutions on the conjunctival vascular permeability in antigen-induced conjunctivitis models.
So, the levels of histamine and SP in the conjunctiva and tears were actually determined in rat antigen-induced conjunctivitis models. Histamine levels in the conjunctiva were decreased by antigen-antibody reaction, but histamine levels in tears were not influenced (data not shown). SP levels in the conjunctiva were decreased and SP levels in tears were increased, suggesting that SP was released from the conjunctiva due to antigen-antibody reaction. Although SP induced the conjunctivitis in guinea pig, histamine level in the conjunctiva had no influence [
4]. SP may be involved in the conjunctivitis not through histamine. In the dermatitis, SP is released from sensory nerve endings and cause histamine release from mast cells [
16]. The visual analogue scale (VAS) score, which evaluated the subjective symptoms of ocular allergy, at the onset of symptoms correlated with tears histamine levels. It have been reported that olopatadine ameliorated the VAS score and decreased the SP levels in tears at the onset of ocular symptoms [
14]. In the present study shows that SP are released in tears and may play a role in rat antigen-induced conjunctivitis models. Olopatadine ophthalmic solutions significantly inhibited the release of SP from the conjunctiva and the amount of SP in tears. On the other hand, tranilast significantly inhibited the amount of SP in tears, and levocabastine failed to inhibit the release of SP from the conjunctiva and the amount of SP in tears. The VAS score at the onset of symptoms correlated with histamine levels in tears [
14]. Although SP also plays an important role in inflammation caused by an increase in vascular permeability, ocular allergy symptoms may be mainly induced by vasoactive amines in rat antigen-induced conjunctivitis models. Therefore, it is suggested that anti-allergic ophthalmic solutions suppressed the conjunctival vascular hyperpermeability in rat antigen-induced conjunctivitis models.
The effects of olopatadine 0.2% to the conjunctival vascular hyperpermeability were showing a strong tendency compared with those of olopatadine 0.1%, especially, the inhibitory of SP release to tears was significantly strong. SP is known itch-associated mediators in dermatitis, however, little is known about the association in AC. It has been reported that oral olopatadine attenuates the amount of scratching behavior and suppress the increased SP levels in the skin lesions in mice chronic inflammatory dermatitis [
10,
11]. It is suggested that chemical mediators including histamine and SP in tears also involved in itching and ocular symptoms. In the clinical study, neuropeptides are up-regulated and their release in nasal fluids has been demonstrated after allergen exposure [
17]. The symptoms of allergic rhinitis are not only with nasal symptoms but also with severe ocular itching. Ocular itching adversely affect the quality of life of patients with allergic rhinitis and allergic rhinoconjunctivitis.
These results indicate that olopatadine ophthalmic solution appears to exert additional SP release inhibition besides its well established dual-action such as selective histamine H1 receptor antagonistic action and human conjunctival mast cell stabilization action. Olopatadine ophthalmic solution may be useful as the first choice for the treatment of AC.
Go to :









 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download