This article has been corrected. See "Erratum: Rhinitis in children less than 6 years of age: current knowledge and challenges" in Volume 2 on page 90.
Abstract
Rhinitis is a disease of the upper airway characterized by runny and/or blocked nose and/or sneezing. Though not viewed as a life threatening condition, it is also recognized to impose significant burden to the quality of life of sufferers and their caretakers and imposes an economic cost to society. Through a PubMed online search of the literature from 2006 to September 2011, this paper aims to review the published literature on rhinitis in young children below the age of 6 years. It is apparent from epidemiology studies that rhinitis in this age group is a relatively common problem. The condition has a heterogenous etiology with classification into allergic and non-allergic rhinitis. Respiratory viral infections may play a role in the pathogenesis of long standing rhinitis, but definitive studies are still lacking. Treatment guidelines for management are lacking for this age group, and is a significant unmet need. Although the consensus is that co-morbidities including otitis media with effusion, adenoidal hypertrophy and asthma, are important considerations of management of these children. Pharmacotherapy is limited for young children especially for those below the age of 2 years. This review underscores the lack of understanding of rhinitis in early childhood and therefore the need for further research in this area.
Rhinitis is an inflammation of the upper airways that is characterised by symptoms of runny (rhinorrhea) and/or blocked nose and/or sneezing occurring for two or more consecutive days and lasting for more than an hour for most days [1, 2]. The Allergic Rhinitis and its Impact on Asthma (ARIA) guideline classifies rhinitis into allergic rhinitis (AR) and non-allergic rhinitis (NAR) based on the presence and absence of allergic sensitization. AR arises due to IgE-mediated mechanisms and is usually characterized with additional symptoms of itchy eyes (conjunctivitis). NAR has multiple possible triggers including infectious, hormonal, occupational, and idiopathic (previously termed as vasomotor rhinitis) [2]. It could well be that rhinitis in young children would involve both components of AR and NAR.
Rhinitis has been trivialized by clinicians as it does not cause fatalities, and has been understudied. More recently, however, rhinitis has been recognized to impose significant morbidity and has a significant impact on the quality of life. It is in fact now considered a considerable global medical burden with a significant economical cost [2]. A recent paediatric survey of children 4-17 years old conducted in the United States showed that rhinitis affects the quality of life (work and sleep) of sufferers and their caregivers [3]. Aside from the direct cost of rhinitis medications, rhinitis also exerts an indirect cost through its co-morbidities such as asthma. An example of this was illustrated by a study by Kang et al that rhinitis in children increased the number of outpatient and hospital visits of asthma sufferers [4].
Two birth cohort studies monitoring the natural history of childhood rhinitis [5, 6] showed a trend of increasing prevalence over time from infancy to adolescents. In these studies, symptoms of rhinitis were already observable from preschool age. Various others studies had also shown that rhinitis symptoms are already prevalent in the first 6 years of life. Except for these studies, it is our impression that rhinitis in early childhood is poorly characterised.
This review aims to summarize our current knowledge and to discuss the gaps in knowledge regarding AR in early childhood.
Literature search was conducted using a PubMed search with the following key terms: 'rhinitis' and 'preschool', and filtering for articles in English and published 2006-2011 (n = 565 articles). We screen titles and abstracts of the search results for studies discussing rhinitis separate from other morbidities, and also for studies discussing subjects <6 years old separately from older children. The screened publications were selected based on the following criteria: (1) Epidemiology and risk factor analysis (n = 45), and (2) therapeutics, intervention and management (n = 13). Most of the initial search results were filtered out as they focussed on co-morbidities such as rhinosinusitis or allergy in general. In addition, most of the other search results touching on rhinitis did not discuss children <6 years old separate from older children.
Additional information on the progression of rhinitis was obtained with the following key search terms: 'rhinitis' and 'natural history' (n = 3). Additionally the search terms 'rhinitis' and 'management guidelines' were included (n = 3).
Fig. 1 illustrates the workflow of the literature search in this review.
Several large professional bodies, ARIA (World Health Organization), American Academy of Asthma Allergy and Clinical Immunology Task Force and British Society of Allergy and Clinical Immunology have published guidelines of the definitions, classification and management of rhinitis [2, 7, 8]. There is currently no management guidelines dedicated to children and this constitutes an unmet need for the clinical management of rhinitis in this age group.
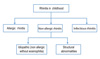
The definition appears to be clear cut. Rhinitis has been defined as a disease of the nasal lining presenting with symptoms of nasal itch, sneezing, runny and/or blocked nose [6-8]. The classification of rhinitis differs slightly between guidelines. The ARIA guideline separates rhinitis into allergic and non-allergic, for which NAR is further classified by the triggering factors into including infectious, hormonal, occupational, and idiopathic (previously termed as vasomotor rhinitis). On the other hand, the British Society for Allergy and Clinical Immunology has classified infectious rhinitis as a separate category from NAR. This classification maybe more appropriate for rhinitis in early childhood where infectious causes may play a predominant role [2, 9]. Fig. 2 is an adaptation of the classification of rhinitis appropriate for childhood. It is both clinically and epidemiologically difficult to differentiate infectious rhinitis from AR in young children [2, 10]. In addition, NAR may also be present with AR, a syndrome termed mixed allergic rhinitis, which may account for 50%-70% of AR cases [11].
Sinusitis often co-exists with rhinitis and the term 'rhinosinusitis' has been coined to reflect this. Nasal polyps are rare in children. Clinical symptoms of discharge (mucopurulent), nasal obstruction, headache and facial pain, and ear pain and pressure are common symptoms of sinusitis [12]. Diagnostic confirmation requires endoscopic examination and CT scan of the sinuses [2]. Endoscopic examination is, however, difficult to perform in young children. Additionally CT scan examination does expose the child to considerable radiation [13]. Hence the diagnosis of sinusitis in children is quite challenging to the clinician and often has to depend on clinical symptoms. Additionally, most of these studies were not accompanied by allergy testing, hence the diagnosis of AR is tentative. It could well be that 'AR symptoms' in this age group may not be sufficiently sensitive for the diagnosis of AR.
Most cross sectional population studies on rhinitis in early childhood have used the validated rhinitis questions from International Study of Allergy and Asthma in Childhood (ISAAC). However, the definitions of AR used in these studies were not entirely identical (Table 1). Some assessed the presence of symptoms indicating AR (sneezing, runny or blocked nose outside of cold or flu). On the other hand, other studies imposed stricter criteria of the requirement of diagnosis by physicians.
The prevalence of rhinitis in preschoolers 0-6 years old various considerably and this may be in part related to the definitions and age groups included in each study. The prevalence of rhinitis symptoms in preschool age groups ranged from 2.8% to 42.7% (Table 1). This wide variation in rhinitis prevalence is very similar to data in school aged children. The ISAAC studies which used the same validated questionnaire also showed a wide variation of rhinitis globally [14]. The studies from Singapore show that the prevalence of rhinitis in preschoolers is substantial with prevalence of 25.3% in the 4 to 6 year age group [15] and a cumulative prevalence of 42.7% in the 2 year age group [16]. These rates are in fact comparable with that reported in the phase III of the ISAAC with rhinitis rates reported at 25.5% for Singapore schoolchildren 6-7 years old [17]. These studies indicate that the prevalence of rhinitis young children may be considerable.
Birth cohort studies provide us with the opportunity to understand the onset and development of rhinitis (Table 2). Besides parental atopy, these studies have provided with information on environmental risk factors for the development of AR such as having siblings [18], environmental tobacco smoke exposure [19,20], traffic pollution [20].
The Isle of Wight birth cohort study has shown that the prevalence of AR continues to increase into adolescence whilst NAR peaks in the preschool age group and then lags behind AR thereafter [6]. Interestingly, gender had a distinct role where NAR was more common in the female gender especially in adolescence. A study from the Paris cohort with follow up till 18 months has shown that the prevalence of AR-like symptoms were 9.1% in these age groups [10].
It therefore appears that rhinitis in young children has a heterogenous etiology. There is a tendency to label these symptoms as AR; however, validation with IgE sensitization, which is often not studied especially in cross sectional is necessary to make a definitive diagnosis.
Intrinsic factors such as male gender, presence of atopy markers (serum IgE and eosinophil count), personal history of eczema or wheeze and allergen sensitization are known risk factors for the development of AR [18, 21-25]. A study by Marinho et al. [26]also showed a dose-response relationship between sensitization by means of specific IgE and skin prick test test results in the risk of rhinoconjunctivitis. Parental, especially maternal, history of allergic symptoms is also a consistently reported risk of AR [21, 23, 27, 28].
Various environmental factors were found to increase the risk of rhinitis in children <6 years old. Pollution factors such as environmental tobacco smoke (ETS) exposure, moulds, and traffic pollution seem to be an important risk of rhinitis. ETS exposure was reported to increase the risk of rhinitis [19, 20]. However, this increased risk was not consistently found [18, 29]. Traffic related pollutions were suggested to be an adjuvant for allergen sensitization [30], which may explain its reported association with increased risk of rhinitis [30, 31].
Exposure to moulds and dust endotoxins in the home may be risk modifiers for rhinitis. A study by Codispoti et al. [18] showed an interesting finding that there was a bimodal shaped relation between dust endotoxin level and the risk of rhinitis. In this study, moderate levels of entotoxin concentration increased the risk of rhinitis. However, there were protective effects against rhinitis at low and high levels of endotoxins instead [18]. Another study of the association between exposure to components of dust and moulds suggested a possible protective factor, though it is only observed in one of the two groups of subjects [32].
Dietary patterns may have protective effects. Several studies have found extended period of breastfeeding to be protective [18, 33]. However, this protective effect was not consistently observed [18, 21, 23, 28]. Though the age of introduction of solid foods in general did not affect the risk of rhinitis [34], early introduction of fish into diet of infants were shown to be correlated to reduced risk of AR [23, 28, 33]. Interestingly a study was done to assess the correlation between maternal vitamin D intake during pregnancy and AR outcome at 5 years old where maternal vitamin D intake from food protected against rhinitis [35].
Respiratory virus infections have been suggested to play a significant role in the inception and exacerbation of asthma [36]. The biological link between respiratory virus infection with wheezing and asthma had been also been studied widely [37]. Birth cohort studies focused on studying asthma have shown that respiratory viruses are commonly detected in infants and young children with rhinorrhoea [38, 39]. Till date, there have not been published data on the role of respiratory virus on the inception of AR or long-term rhinitis symptoms. Regardless, the same set of viruses suggested related to asthma, such as human rhinovirus, respiratory synctitial virus, human parainfluenza virus, human influenza virus and human coronavirus, have been detected in infants with upper respiratory tract infection and rhinorrhea [40]. Since respiratory viruses have been shown to cause inflammatory changes in the upper airway as has been seen in the lower airway [41], it is plausible for respiratory virus infections to play a role in the development of AR or NAR. An ongoing birth cohort study in Singapore (GUSTO; http://www.gusto.sg/) will focus on this role of early respiratory viruses infections in development of rhinitis including AR.
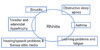
Besides its direct effect on the quality of life, rhinitis has significant co-morbid disorders (summarised in Fig. 3). The ARIA recommendations emphasizes the concept of treating upper and lower airway disorders as 'one airway, one disease' which includes a holistic approach towards the management of co-morbidities such as asthma, sinusitis, otitis media and conjunctivitis [42].
Adenoidal hypertrophy may be associated with AR [43]. Conversely adenoidal hypertrophy may mimic the symptoms of AR and therefore should always be considered in young children diagnosed with a diagnosis of AR [7]. Otitis media with effusion is often a co-morbidity of AR and NAR and should be evaluated in all young children with rhinitis. Recent onset otitis media with effusion in children [44] or occurring in the first 2 years of life [45] is associated with rhinitis at 6 years of age.
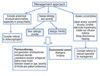
The management of rhinitis and its co-morbidities in young children may require a multidisciplinary approach involving the paediatrician or paediatric allergist and the otolaryngologist. Fig. 4 is a suggested management strategy for young children with rhinitis.
A significant unmet need lies in the pharmacological armamentarum that is approved for use in children less than 2 years of age. First generation antihistamines are not recommended due to its sedative effect [2, 7, 8]. A second generation antihistamine, fexofenadine, had been tested to be safe in children 2 years old or older for management of AR [46] and from 6 months for chronic urticaria . Cetirizine and desloratidine have approval of use for AR in children as young as 6 months of age.
Intranasal corticosteroids such as mometasone furoate and mometasone diproprionate have age limit approval from 2 years and above (with the exception for EMEA for mometasone diproprionate where approval is for 6 years and above). Intranasal application of medications may also be challenging in terms of the child's co-operation with the application. In a recent study in Singaporean children about a quarter of children prescribed topical nasal medications refused to comply with therapy [47]. Montelukast is an additional anti-inflammatory agent that is approved for use for AR in children above the age of 6 months. However, a meta-analysis on very limited data in children, on comparative data between antihistamines, leukotriene receptor antagonists and intranasal corticosteroids showed that intranasal corticosteroids were the most effective for relief of AR [48].
Non pharmacological therapy with saline nasal rinses has also shown modest improvement in rhinitis symptoms in those above the age of 6 years [49].
Allergen immunotherapy is another therapeutic strategy that is attractive in that it reduces new sensitizations, reduces the risk of asthma and has a long term carry over effect even after cessation of therapy [2].
Rhinitis in young children is common with significant morbidity. There are significant gaps in our knowledge in terms of classification and phenotyping rhinitis in this age group. There are many unmet needs in the management and treatment guidelines are lacking. The WHO ARIA guidelines currently devote only 4 pages to childhood rhinitis in a document of 153 pages including references [2].
Figures and Tables
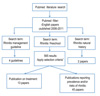 | Fig. 1The workflow of the literature review. *Criteria: 1. Study analyzed rhinitis separately from other disease conditions; 2. Study analyzes subjects aged 6 and below separately from subjects aged >6. |
References
1. International Rhinitis Management Working Group. International Consensus Report on the diagnosis and management of rhinitis. Allergy. 1994. 49:1–34.
2. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica GW, Van Weel C, Agache I, Aït-Khaled N, Bachert C, Blaiss MS, Bonini S, Boulet LP, Bousquet PJ, Camargos P, Carlsen KH, Chen Y, Custovic A, Dahl R, Demoly P, Douagui H, Durham SR, Van Wijk RG, Kalayci O, Kaliner MA, Kim YY, Kowalski ML, Kuna P, Le LT, Lemiere C, Li J, Lockey RF, Mavale-Manuel S, Meltzer EO, Mohammad Y, Mullol J, Naclerio R, O'Hehir RE, Ohta K, Ouedraogo S, Palkonen S, Papadopoulos N, Passalacqua G, Pawankar R, Popov TA, Rabe KF, Rosado-Pinto J, Scadding GK, Simons FE, Toskala E, Valovirta E, Van Cauwenberge P, Wang DY, Wickman M, Yawn BP, Yorgancioglu A, Yusuf OM, Zar H, Annesi-Maesano I, Bateman ED, Ben Kheder A, Boakye DA, Bouchard J, Burney P, Busse WW, Chan-Yeung M, Chavannes NH, Chuchalin A, Dolen WK, Emuzyte R, Grouse L, Humbert M, Jackson C, Johnston SL, Keith PK, Kemp JP, Klossek JM, Larenas-Linnemann D, Lipworth B, Malo JL, Marshall GD, Naspitz C, Nekam K, Niggemann B, Nizankowska-Mogilnicka E, Okamoto Y, Orru MP, Potter P, Price D, Stoloff SW, Vandenplas O, Viegi G, Williams D. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA2LEN and AllerGen). Allergy. 2008. 63:Suppl 86. 8–160.
3. Meltzer EO, Blaiss MS, Derebery MJ, Mahr TA, Gordon BR, Sheth KK, Simmons AL, Wingertzahn MA, Boyle JM. Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. J Allergy Clin Immunol. 2009. 124:S43–S70.

4. Kang HY, Park CS, Bang HR, Sazonov V, Kim CJ. Effect of allergic rhinitis on the use and cost of health services by children with asthma. Yonsei Med J. 2008. 49:521–529.

5. Keil T, Bockelbrink A, Reich A, Hoffmann U, Kamin W, Forster J, Schuster A, Willich SN, Wahn U, Lau S. The natural history of allergic rhinitis in childhood. Pediatr Allergy Immunol. 2010. 21:962–969.

6. Kurukulaaratchy RJ, Karmaus W, Raza A, Matthews S, Roberts G, Arshad SH. The influence of gender and atopy on the natural history of rhinitis in the first 18 years of life. Clin Exp Allergy. 2011. 41:851–859.

7. Wallace DV, Dykewicz MS, Bernstein DI, Blessing-Moore J, Cox L, Khan DA, Lang DM, Nicklas RA, Oppenheimer J, Portnoy JM, Randolph CC, Schuller D, Spector SL, Tilles SA. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008. 122:S1–S84.

8. Scadding GK, Durham SR, Mirakian R, Jones NS, Leech SC, Farooque S, Ryan D, Walker SM, Clark AT, Dixon TA, Jolles SR, Siddique N, Cullinan P, Howarth PH, Nasser SM. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2008. 38:19–42.

9. Sih T, Mion O. Allergic rhinitis in the child and associated comorbidities. Pediatr Allergy Immunol. 2010. 21:e107–e113.

10. Herr M, Clarisse B, Nikasinovic L, Foucault C, Le Marec AM, Giordanella JP, Just J, Momas I. Does allergic rhinitis exist in infancy? Findings from the PARIS birth cohort. Allergy. 2011. 66:214–221.

11. Bernstein JA. Allergic and mixed rhinitis: Epidemiology and natural history. Allergy Asthma Proc. 2010. 31:365–369.

12. Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, Bachert C, Baraniuk J, Baroody FM, Benninger MS, Brook I, Chowdhury BA, Druce HM, Durham S, Ferguson B, Gwaltney JM, Kaliner M, Kennedy DW, Lund V, Naclerio R, Pawankar R, Piccirillo JF, Rohane P, Simon R, Slavin RG, Togias A, Wald ER, Zinreich SJ. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004. 114:155–212.

13. Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007. 357:2277–2284.
14. Björkstén B, Clayton T, Ellwood P, Stewart A, Strachan D. Worldwide time trends for symptoms of rhinitis and conjunctivitis: Phase III of the International Study of Asthma and Allergies in Childhood. Pediatr Allergy Immunol. 2008. 19:110–124.

15. Tan TN, Shek LP, Goh DY, Chew FT, Lee BW. Prevalence of asthma and comorbid allergy symptoms in Singaporean preschoolers. Asian Pac J Allergy Immunol. 2006. 24:175–182.
16. Tan TN, Lim DL, Lee BW, Van Bever HP. Prevalence of allergy-related symptoms in Singaporean children in the second year of life. Pediatr Allergy Immunol. 2005. 16:151–156.

17. Wang XS, Tan TN, Shek LP, Chng SY, Hia CP, Ong NB, Ma S, Lee BW, Goh DY. The prevalence of asthma and allergies in Singapore; data from two ISAAC surveys seven years apart. Arch Dis Child. 2004. 89:423–426.

18. Codispoti CD, Levin L, LeMasters GK, Ryan P, Reponen T, Villareal M, Burkle J, Stanforth S, Lockey JE, Khurana Hershey GK, Bernstein DI. Breast-feeding, aeroallergen sensitization, and environmental exposures during infancy are determinants of childhood allergic rhinitis. J Allergy Clin Immunol. 2010. 125:1054–1060.e1.

19. Johansson A, Ludvigsson J, Hermansson G. Adverse health effects related to tobacco smoke exposure in a cohort of three-year olds. Acta Paediatr. 2008. 97:354–357.

20. Dong GH, Ma YN, Ding HL, Jin J, Cao Y, Zhao YD, He QC. Housing characteristics, home environmental factors and respiratory health in 3945 pre-school children in China. Int J Environ Health Res. 2008. 18:267–282.

21. Marinho S, Simpson A, Lowe L, Kissen P, Murray C, Custovic A. Rhinoconjunctivitis in 5-year-old children: a population-based birth cohort study. Allergy. 2007. 62:385–393.
22. Schäfer T, Stieger B, Polzius R, Krauspe A. Associations between cat keeping, allergen exposure, allergic sensitization and atopic diseases: results from the Children of Lübeck Allergy and Environment Study (KLAUS). Pediatr Allergy Immunol. 2009. 20:353–357.

23. Alm B, Goksör E, Thengilsdottir H, Pettersson R, Möllborg P, Norvenius G, Erdes L, Åberg N, Wennergren G. Early protective and risk factors for allergic rhinitis at age 4½ yr. Pediatr Allergy Immunol. 2011. 22:398–404.

24. Almqvist C, Li Q, Britton WJ, Kemp AS, Xuan W, Tovey ER, Marks GB. Early predictors for developing allergic disease and asthma: examining separate steps in the 'allergic march'. Clin Exp Allergy. 2007. 37:1296–1302.

25. Brockow I, Zutavern A, Hoffmann U, Grübl A, von Berg A, Koletzko S, Filipiak B, Bauer CP, Wichmann HE, Reinhardt D, Berdel D, Krämer U, Heinrich J. Early allergic sensitizations and their relevance to atopic diseases in children aged 6 years: results of the GINI study. J Investig Allergol Clin Immunol. 2009. 19:180–187.
26. Marinho S, Simpson A, Söderström L, Woodcock A, Ahlstedt S, Custovic A. Quantification of atopy and the probability of rhinitis in preschool children: a population-based birth cohort study. Allergy. 2007. 62:1379–1386.

27. Siriaksorn S, Suchaitanawanit S, Trakultivakorn M. Allergic rhinitis and immunoglobulin deficiency in preschool children with frequent upper respiratory illness. Asian Pac J Allergy Immunol. 2011. 29:73–77.

28. Virtanen SM, Kaila M, Pekkanen J, Kenward MG, Uusitalo U, Pietinen P, Kronberg-Kippilä C, Hakulinen T, Simell O, Ilonen J, Veijola R, Knip M. Early introduction of oats associated with decreased risk of persistent asthma and early introduction of fish with decreased risk of allergic rhinitis. Br J Nutr. 2010. 103:266–273.

29. Dong GH, Ren WH, Wang D, Yang ZH, Zhang PF, Zhao YD, He QC. Exposure to secondhand tobacco smoke enhances respiratory symptoms and responses to animals in 8,819 children in kindergarten: results from 25 districts in northeast China. Respiration. 2011. 81:179–185.

30. Morgenstern V, Zutavern A, Cyrys J, Brockow I, Koletzko S, Krämer U, Behrendt H, Herbarth O, von Berg A, Bauer CP, Wichmann HE, Heinrich J. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med. 2008. 177:1331–1337.

31. Zuraimi MS, Tham KW, Chew FT, Ooi PL, Koh D. Home air-conditioning, traffic exposure, and asthma and allergic symptoms among preschool children. Pediatr Allergy Immunol. 2011. 22:e112–e118.

32. Tischer C, Gehring U, Chen CM, Kerkhof M, Koppelman G, Sausenthaler S, Herbarth O, Schaaf B, Lehmann I, Krämer U, Berdel D, von Berg A, Bauer CP, Koletzko S, Wichmann HE, Brunekreef B, Heinrich J. Respiratory health in children, and indoor exposure to (1,3)-β-D-glucan, EPS mould components and endotoxin. Eur Respir J. 2011. 37:1050–1059.

33. Kull I, Bergström A, Lilja G, Pershagen G, Wickman M. Fish consumption during the first year of life and development of allergic diseases during childhood. Allergy. 2006. 61:1009–1015.

34. Zutavern A, Brockow I, Schaaf B, von Berg A, Diez U, Borte M, Kraemer U, Herbarth O, Behrendt H, Wichmann HE, Heinrich J. Timing of solid food introduction in relation to eczema, asthma, allergic rhinitis, and food and inhalant sensitization at the age of 6 years: results from the prospective birth cohort study LISA. Pediatrics. 2008. 121:e44–e52.

35. Erkkola M, Kaila M, Nwaru BI, Kronberg-Kippilä C, Ahonen S, Nevalainen J, Veijola R, Pekkanen J, Ilonen J, Simell O, Knip M, Virtanen SM. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clin Exp Allergy. 2009. 39:875–882.

36. Gern JE. Viral respiratory infection and the link to asthma. Pediatr Infect Dis J. 2004. 23:S78–S86.

37. Tauro S, Su YC, Thomas S, Schwarze J, Matthaei KI, Townsend D, Simson L, Tripp RA, Mahalingam S. Molecular and cellular mechanisms in the viral exacerbation of asthma. Microbes Infect. 2008. 10:1014–1023.

38. Bisgaard H, Hermansen MN, Bønnelykke K, Stokholm J, Baty F, Skytt NL, Aniscenko J, Kebadze T, Johnston SL. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010. 341:c4978.

39. Lee KK, Hegele RG, Manfreda J, Wooldrage K, Becker AB, Ferguson AC, Dimich-Ward H, Watson WT, Chan-Yeung M. Relationship of early childhood viral exposures to respiratory symptoms, onset of possible asthma and atopy in high risk children: the Canadian Asthma Primary Prevention Study. Pediatr Pulmonol. 2007. 42:290–297.

40. van Benten I, Koopman L, Niesters B, Hop W, van Middelkoop B, de Waal L, van Drunen K, Osterhaus A, Neijens H, Fokkens W. Predominance of rhinovirus in the nose of symptomatic and asymptomatic infants. Pediatr Allergy Immunol. 2003. 14:363–370.

41. van Benten IJ, van Drunen CM, Koevoet JL, Koopman LP, Hop WC, Osterhaus AD, Neijens HJ, Fokkens WJ. Reduced nasal IL-10 and enhanced TNFalpha responses during rhinovirus and RSV-induced upper respiratory tract infection in atopic and non-atopic infants. J Med Virol. 2005. 75:348–357.
42. Bachert C, Vignola AM, Gevaert P, Leynaert B, Van Cauwenberge P, Bousquet J. Allergic rhinitis, rhinosinusitis, and asthma: one airway disease. Immunol Allergy Clin North Am. 2004. 24:19–43.

43. Modrzynski M, Zawisza E. An analysis of the incidence of adenoid hypertrophy in allergic children. Int J Pediatr Otorhinolaryngol. 2007. 71:713–719.

44. MacIntyre EA, Chen CM, Herbarth O, Borte M, Schaaf B, Krämer U, von Berg A, Wichmann HE, Heinrich J. Early-life otitis media and incident atopic disease at school age in a birth cohort. Pediatr Infect Dis J. 2010. 29:e96–e99.

45. Thomson JA, Widjaja C, Darmaputra AA, Lowe A, Matheson MC, Bennett CM, Allen K, Abramson MJ, Hosking C, Hill D, Dharmage SC. Early childhood infections and immunisation and the development of allergic disease in particular asthma in a high-risk cohort: A prospective study of allergy-prone children from birth to six years. Pediatr Allergy Immunol. 2010. 21:1076–1085.

46. Hampel F, Kittner B, van Bavel J. Safety and tolerability of fexofenadine hydrochloride, 15 and 30 mg, twice daily in children aged 6 months to 2 years with allergic rhinitis. Ann Allergy Asthma Immunol. 2007. 99:549–554.

47. Wong IY, Soh SE, Chng SY, Shek LP, Goh DY, Van Bever HP, Lee BW. Compliance with topical nasal medication--an evaluation in children with rhinitis. Pediatr Allergy Immunol. 2010. 21:1146–1150.
48. Wilson AM, O'Byrne PM, Parameswaran K. Leukotriene receptor antagonists for allergic rhinitis: a systematic review and meta-analysis. Am J Med. 2004. 116:338–344.

49. Slapak I, Skoupá J, Strnad P, Horník P. Efficacy of isotonic nasal wash (seawater) in the treatment and prevention of rhinitis in children. Arch Otolaryngol Head Neck Surg. 2008. 134:67–74.

50. Liukkonen K, Virkkula P, Aronen ET, Kirjavainen T, Pitkäranta A. All snoring is not adenoids in young children. Int J Pediatr Otorhinolaryngol. 2008. 72:879–884.

51. Peroni DG, Piacentini GL, Bodini A, Boner AL. Preschool asthma in Italy: prevalence, risk factors and health resource utilization. Respir Med. 2009. 103:104–108.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download