Abstract
A phytochemical investigation of Allium macrostemon Bunge (Liliaceae) afforded the new pregnane steroidal glycoside, named allimacroside F (1), along with three known glycosides, benzyl-O-α-L-rhamnopy-ranosyl-(1 → 6)-β-D-glucopyranoside (2), phenylethyl-O-α-L-rhamnopyranosyl-(1 → 6)-β-D-glucopyranoside (3), (Z)-3-hexenyl-O-α-L-rhamnopyranosyl-(1 →6)-β-D-glucopyranoside (4). The identification and structural elucidation of a new compound (1) was carried out based on spectral data analyses (1H-NMR,13C-NMR,1H–1H COSY, HSQC, HMBC, and NOESY) and HR-FAB-MS.
Go to : 
References
(1). Usui A.., Matsuo Y.., Tanaka T.., Ohshima K.., Fukuda S.., Mine T.., Nakayama H.., Ishimaru K.Nat. Prod. Commun. 2017. 12:89–91.
(2). Jiangsu New Medical College. Dictionary of Chinese Drugs; Shanghai Science and Technological Publisher: China,. 2001. , p. 2642.
(3). Sobolewska D.., Michalska K.., Podolak I.., Grabowska K.Phytochem. Rev. 2016. 15:1–35.
(4). Xie W.., Zhang Y.., Wang N.., Zhou H.., Du L.., Ma X.., Shi X.., Cai G.Eur. J. Pharmacol. 2008. 599:159–165.
(5). Peng J.., Yao X.., Okada Y.., Okuyama T.Chem. Pharm. Bull. 1994. 42:2180–2182.
(6). Chen H. -F.., Wang N. -L.., Sun H. -L.., Yang B. -F.., Yao X. -S. J.Asian Nat. Prod. Res. 2006. 8:21–28.
(7). Chen H.., Wang G.., Wang N.., Yang M.., Wang Z.., Wang X.., Yao X.Pharmazie. 2007. 62:544–548.
(8). Cheng S. -B.., Wang Y.., Zhang Y. -F.., Wang Y.Zhong cao yao. 2013. 44:1078–1081.
(9). Kim Y. S.., Suh W. S.., Park K. J.., Choi S. U.., Lee K. R.Steroids. 2017. 118:41–46.
(10). Hamerski L.., Bomm M. D.., Silva D. H. S.., Young M. C. M.., Furlan M.., Eberlin M. N.., Castro-Gamboa I.., Cavalheiro A. J.., da Silva Bolzani V.Phytochemistry. 2005. 66:1927–1932.
(11). Umehara K.., Hattori I.., Miyase T.., Ueno A.., Hara S.., Kageyama C.Chem. Pharm. Bull. 1988. 36:5004–5008.
(12). Inagaki J.., Watanabe N.., Moon J. -H.., Yagi A.., Sakata K.., Ina K.., Luo S.Biosci. Biotechnol. Biochem. 1995. 59:738–739.
(13). Kishida M.., Fujii M.., Ida Y.Heterocycles. 2005. 65:2127–2137.
(14). Yokosuka A.., Mimaki Y.., Sashida Y. J.Nat. Prod. 2000. 63:1239–1243.
(15). Temraz A.., El Gindi O. D.., Kadry H. A.., De Tommasi N.., Braca A.Phytochemistry. 2006. 67:1011–1018.
Go to : 
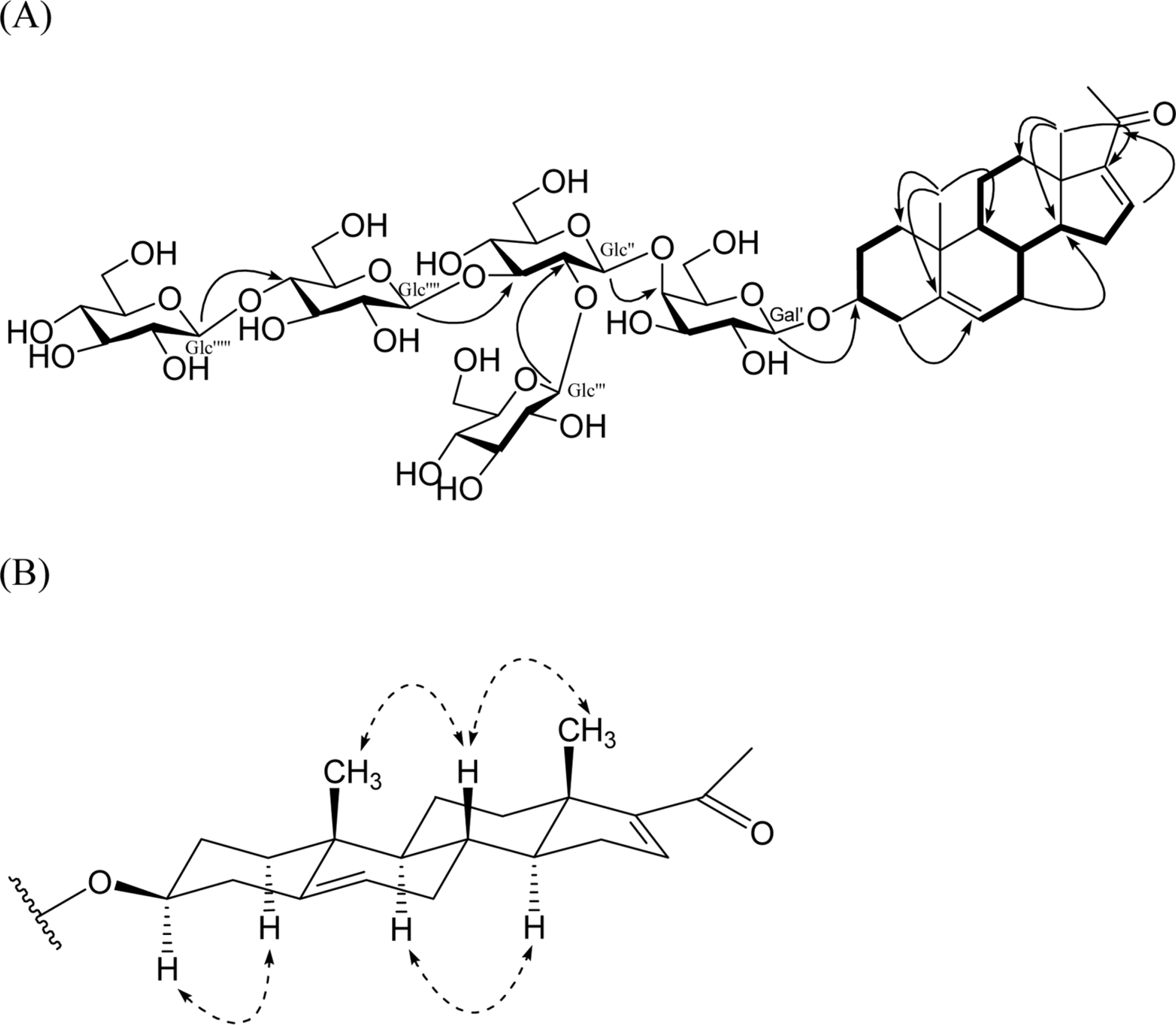 | Fig. 2.Key HMBC (HC), 1H-1H COSY (—) correlations 1 (A), and NOESY () correlations of 1 (B). |
Table 1.
1H and 13C NMR data of 1 in Pyridine-d5. (δ in ppm, 700 MHz for 1H and 175 MHz for 13C)a




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download