Abstract
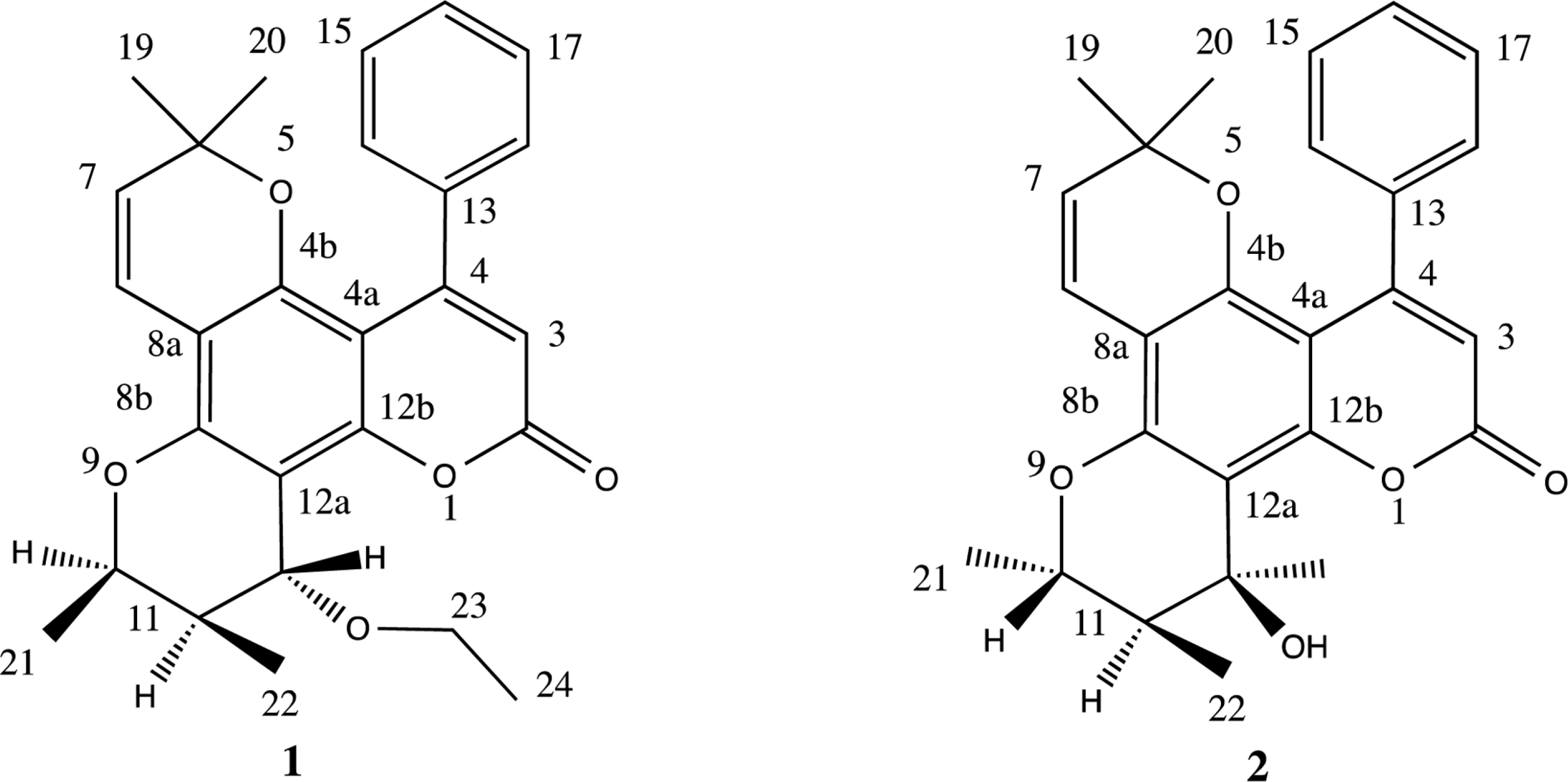
Callophylum symingtonianum (Guttiferae), an evergreen broad-leaved tree that usually grows in hill forests, can be found distributed in the Malay Peninsula. The barks, leaves, flowers and seeds is often used medicinally to treat diarrhea and rheumatism. In the present study, we isolated two inophyllum type coumarins, 12-O-ethylinophyllum D (1) and iso-soulattrolide (2) from the stembarks of C. symingtonianum together with their antibacterial activity. The compounds were isolated by chromatographic methods on a silica gel. The structures were established by spectroscopic methods including UV, IR, (1D and 2D) NMR and mass spectrometry as well as by comparison with several literature sources. The antibacterial activity of those compounds was tested using a disc-diffusion assay against Staphylococcus aureus, Bacillus cereus, Escherichia coli and Pseudomonas aeruginosa. Both compound exhibited mild inhibition against P. aeruginosa with both 111 µg/ml MIC value. Compound 2 also inhibits S. aureus with 25 µg/ml MIC value.
Go to : 
References
(1). Stevens P. F. J.Arnold Arbor. 1980. 61:117–424.
(2). Orwa C.., Mutua A.., Kindt R.., Jamnadass R. H.., Simons A.Agroforestree Database: a tree reference and selection guide version 4.0. 2009.
(3). Zaidan M. R. S.., Noor Rain A.., Badrul A. R.., Adlin A.., Norazah A.., Zakiah I.Trop. Biomed. 2005. 22:165–170.
(4). Wiegand I.., Hilpert K.., Hancock R. E.Nat. Protoc. 2008. 3:163–175.
(5). Zou J.., Wu J.., Liu S. -Z.., Zhao W.-M. Helv. Chim. Acta. 2010. 93:1812–1821.
(6). Gunasekera S. P.., Jayatilake G. S.., Selliah S. S.., Sultanbawa M. U. S. J.Chem. Soc. Perkin Trans. 1. 1977. 13:1505–1511.
(7). Bandara B. M. R.., Dharmaratne H. R. W.., Sotheeswaran S.., Balasubramaniam S.Phytochemistry. 1986. 25:425–428.
(8). Patil A. D.., Freyer A. J.., Eggleston D. S.., Haltiwanger R. C.., Bean M. F.., Taylor P. B.., Caranfa M. J.., Breen A. L.., Bartus H. R.., Johnson R. K.., Hertzberg R. P.., Westley J. W. J.Med. Chem. 1993. 36(4131–4138):4131–4138.
(9). Kawazu K.., Ohigashi H.., Mitsui T.Tetrahedron Lett. 1968. 9:2383–2385.
(10). Kawazu K.., Ohigashi H.., Takahashi N.., Mitsui T.Bull. Inst. Chem. Res. 1972. 50:160–167.
(11). Shi X.., Attygalle A. B.., Liwo A.., Hao M. -H.., Meinwald J.., Dharmaratne H. R. W.., Wanigasekera W. M. A. P. J.Org. Chem. 1998. 63:1233–1238.
Go to : 
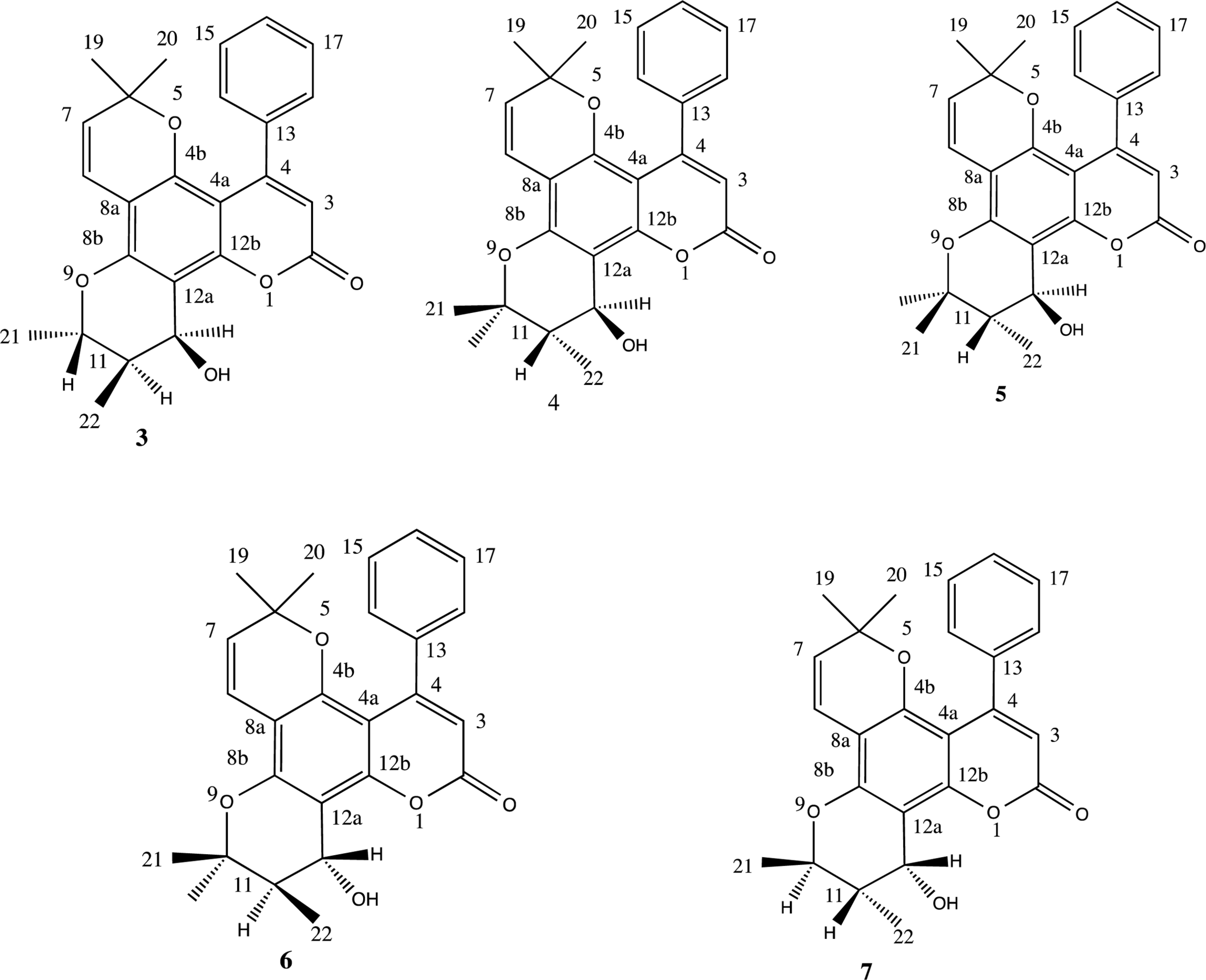
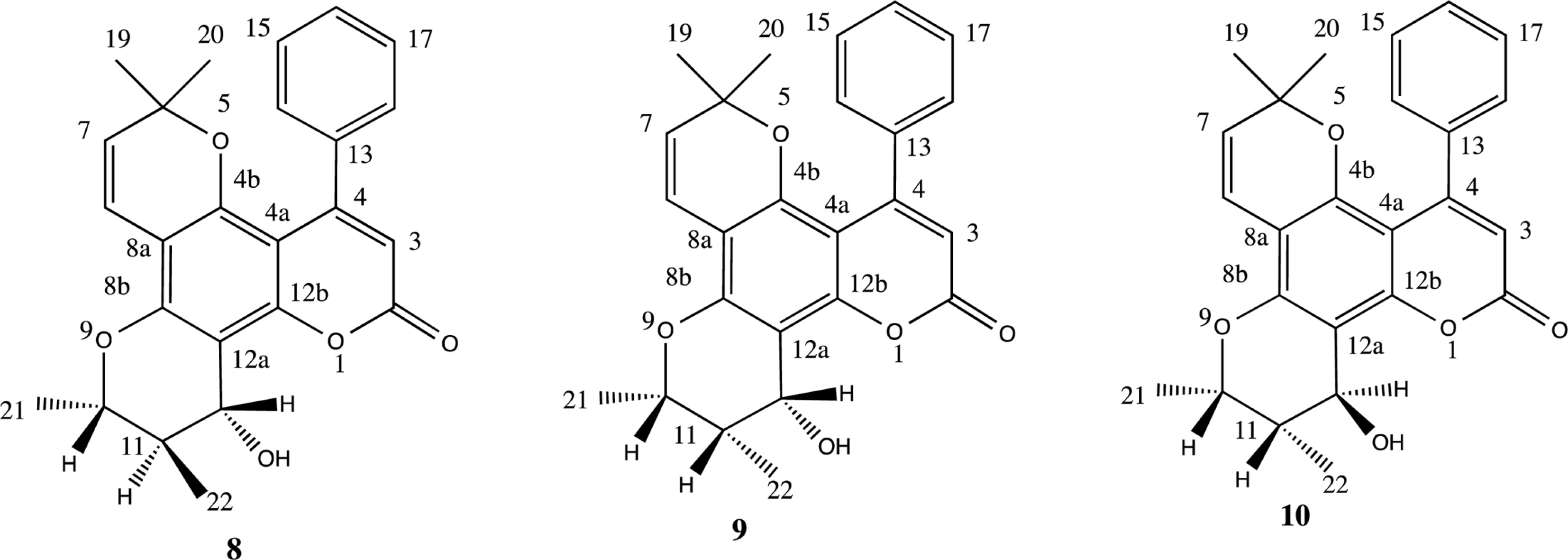
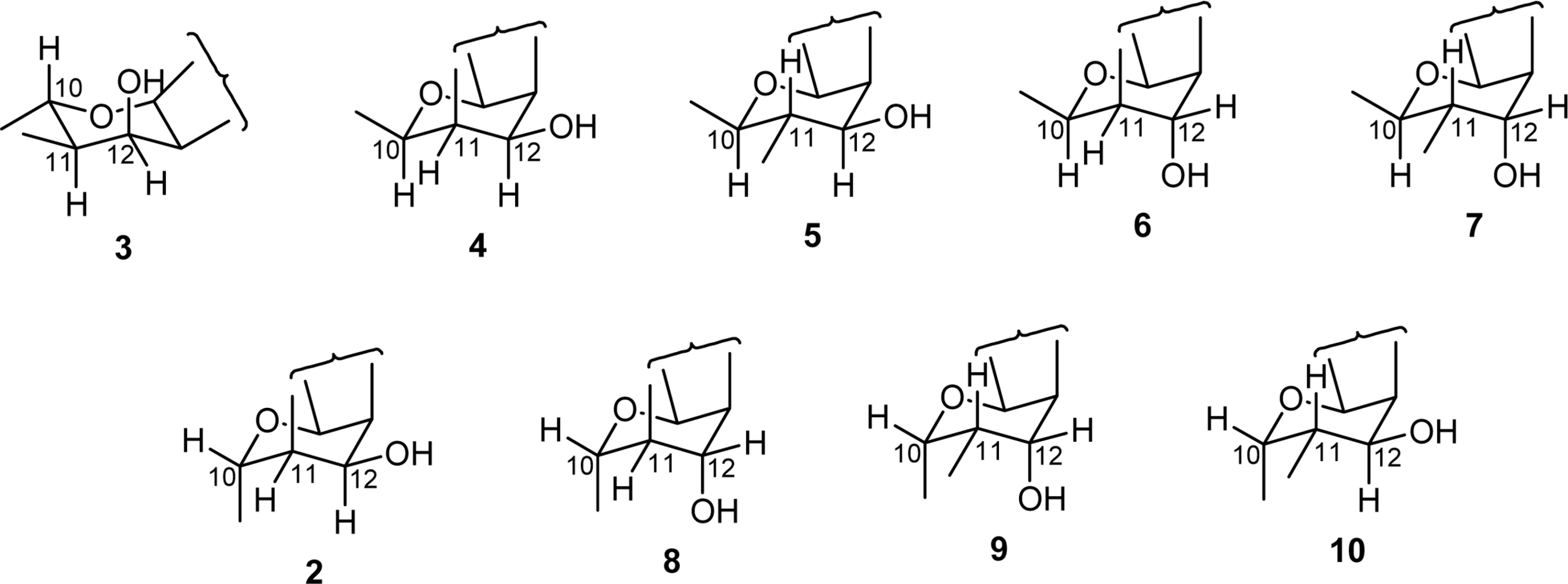 | Fig. 4.Chromanol ring conformational structures of reported inophyllums 3 – 7 and probable structures of the compound 2 (2, 8 – 10). |
Table 1.
1H NMR (60, 100 & 400 MHz) spectroscopic data of compounds 1 – 7 (CDCl3, δ, ppm, J/Hz)
| Proton | 1∗ | 2∗ | Soulattrolide (3)∗∗ 6 | Inophyllum A (4)∗∗∗6 | Inophyllum B (5)∗ 8 | Inophyllum D (6)∗5 | Inophyllum P (7)∗ 8 |
|---|---|---|---|---|---|---|---|
| 3 | 5.96 (1H, s) | 5.94 (1H, s) | 5.94 (1H, s) | 5.96 (1H, s) | 5.97 (1H, s) | 5.98 (1H, s) | 5.97 (1H, s) |
| 7 | 5.33 (1H, d, J=10.0) | 5.30 (1H, d, J = 10.0) | 5.35 (1H, d, J = 10.0) | 5.37 (1H, d, J = 10.0) | 5.37 (1H, d, J = 10.0) | 5.36 (1H, d, J = 10.0) | 5.37 (1H, d, J = 10.0) |
| 8 | 6.54 (1H, d, J = 10.0) | 6.51 (1H, d, J = 10.0) | 6.53 (1H, d, J = 10.0) | 6.55 (1H, d, J = 10.0) | 6.53 (1H, d, J = 10.0) | 5.66 (1H, d, J = 10.0) | 6.54 (1H, d, J = 10.0) |
| 10 | 4.56 (1H, dq, J = 6.4, 1.6) | 4.63 (1H, dq, J = 6.4, 1.6) | 4.31 (1H, m, J = 7.0, 10.0) | 4.43 (1H, m, J = 7.0, 3.3) | ) 3.96 (1H, dq, J = 6.4, 9.1) | 4.56 (1H, dq, J = 6.7, 2.0) | 4.29 (1H, dq, J = 6.3, 10.6) |
| 11 | 2.07 (1H, ddq, J = 7.2, 2.0, 2.0) | , 3.01 (1H, ddq, J = 7.2, 2.0, 2.0) | 1.78 (1H, m, J = 3.2, 10.0) | 2.27 (1H, m) | 1.97 (1H, ddq, J = 6.8, 9.1 7.8) | , 1.99 (1H, ddq, J = 7.2, 2.0, 2.0) | 1.79 (1H, ddq, J = 3.3, 7.0, 10.6) |
| 12 | 4.54 (1H, d, J = 2.0) | 4.88 (1H, d, J = 1.4) | 5.04 (1H, d, J = 3.2) | 5.17 (1H, d, J = 5.4) | 4.79 (1H, d, J = 7.8) | 4.95 (1H, d, J = 2.0) | 5.04 (1H, d, J = 3.3) |
| 14 | 7.22 (1H, m) | 7.23 (1H, m) | 7.31–7.33 (1H, m) | ||||
| 15 | 7.35 (1H, m) | 7.35 (1H, m) | 7.29–7.30 (1H, m) | ||||
| 16 | 7.35 (1H, m) | 7.35 (1H, m) | 7.30 (5H, m) | 7.30 (5H, m) | 7.41, 7.31 (5H, m) | 7.31–7.33 (1H, m) | 7.42, 7.30 (5H, m) |
| 17 | 7.35 (1H, m) | 7.35 (1H, m) | 7.29–7.30 (1H, m) | ||||
| 18 | 7.22 (1H, m) | 7.23 (1H, m) | 7.31–7.33 (1H, m) | ||||
| 19 20 | 0.90 (3H, s) 0.93 (3H, s) | 0.89 (6H, s) | 0.93 (6H, s) | 0.94 (6H, s) | 0.97 (3H, s) 0.91 (3H, s) | 0.95 (6H, s) | 0.93 (6H, s) |
| 21 | 1.42 (3H, d, J = 6.8) | 1.56 (3H, d, J = 6.4) | 1.44 (3H, d, J = 7.0) | 1.43 (3H, d, J = 7.0) | 1.47 (3H, d, J = 6.4) | 1.45 (3H, d, J = 6.7) | 1.44 (3H, d, J = 6.3) |
| 22 | 0.79 (3H, d, J = 7.2) | 0.90 (3H, d, J = 7.6) | 1.16 (3H, d, J = 7.2) | 1.17 (3H, d, J = 7.2) | 1.18 (3H, d, J = 6.8) | 0.83 (3H, d, J = 7.2) | 1.17 (3H, d, J = 7.0) |
| 23 | 3.85 (2H, q, J = 7.2) | — | — | — | — | — | — |
| 24 | 1.28 (3H, t, J = 6.8) | — | — | — | — | — | — |
Table 2.
13C NMR (100 MHz) spectroscopic data of compounds 1, 2, 4 – 7 (CDCl3, δ, ppm)
Table 3.
Epimers and diastereomers of the known inophyllums 4 – 7∗
Table 4.
Antibacterial activity of the isolated compounds




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download