Abstract
The aim of this study was to measure and compare polyphenol content, antioxidant, and antiinflammatory activity of six halophytes (Limonium tetragonum, Suaeda glauca, Suaeda japonica, Salicornia europaea, Triglochin maritimum, and Sonchus brachyotus). Depending on the total polyphenol content, the plants were categorized into two groups: (1) a high total polyphenol content group that included L. tetragonum, S. brachyotus, and S. europaea, and, (2) a low total polyphenol content group consisting of S. glauca, T. maritima, and S. japonica. Antioxidant activity was evaluated using DPPH and hydroxyl radical scavenging assays, and by measuring ROS. Anti-inflammatory activity was evaluated by measuring NO and PGE2. L. tetragonum and S. brachyotus, that have high polyphenol content, also showed strong antioxidant activity. In addition, L. tetragonum, S. brachyotus, and S. europaea showed good anti-inflammatory activity. Consequently, the total polyphenol content was thought to be related to antioxidant and anti-inflammatory activity. Therefore, S. brachyotus and L. tetragonum are good candidates for use in pharmaceuticals and functional foods.
Go to : 
References
(1). El Shaer, H. M. Potential of halophytes as animal fodder in Egypt. in: Lieth H.., Mochtchenko M.Part II: Chemical Contents. Cash Crop Halophytes: Recent Studies; Kluwer Academic Publishers: London,. 2003. 111–119.
(2). Kim N. -H.., Heo J. -D.., Kim T. B.., Rho J. -R.., Yang M. H.., Jeong E. J.Biol. Pharm. Bull. 2016. 39:1022–1028.
(3). An R. -B.., Sohn D. -H.., Jeong G. -S.., Kim Y.-C. Arch. Pharm. Res. 2008. 31:594–597.
(4). Lee S. -G.., Kim J. -B.., Kang H.Trop. J. Pharm. Res. 2016. 15:1175–1181.
(5). Choi J. -I.., Kim Y. -J.., Kim J. -H.., Song B. -S.., Yoon Y.., Byun M. -W.., Kwon J. -H.., Chun S. -S.., Lee J. -W. J.Korean Soc. Food Sci. Nutr. 2009. 38:131–135.
(6). Kang H.., Koppula S.., Kim H. -K.., Park T. -K.Trop. J. Pharm. Res. 2013. 12:351–356.
(7). Lellau T. F.., Liebezeit G.Pharm. Biol. 2003. 41:293–300.
(8). Lellau T. F.., Liebezeit G.Mar. Biodivers. 2003. 32:177–181.
(9). Nugroho A.., Kim M. -H.., Lee C. M.., Choi J. S.., Lee S.., Park H.-J. Nat. Prod. Sci. 2012. 18:39–46.
(10). Xia D. -Z.., Yu X. -F.., Zhu Z. -Y.., Zou Z.-D. Nat. Prod. Res. 2011. 25:1893–1901.
(11). Cho J. -Y.., Yang X.., Park K. -H.., Park H. J.., Park S. -Y.., Moon J. -H.., Ham K.-S. Food Sci. Biotechnol. 2013. 22:1547–1557.
(12). Lee J. I.., Kong C. -S.., Jung M. E.., Hong J. W.., Lim S. Y.., Seo Y.Biotechnol. Bioprocess Eng. 2011. 16:992–999.
(13). Qiu P.., Wang Q. -Z.., Yin M.., Wang M.., Zhao Y. -Y.., Shan Y.., Feng X.Zhong Yao Cai. 2015. 38:751–753.
(14). Agati G.., Azzarello E.., Pollastri S.., Tattini M.Plant Sci. 2012. 196:67–76.
(15). Sun L.., Zhang J.., Lu X.., Zhang L.., Zhang Y.Food Chem. Toxicol. 2011. 49:2689–2696.
(16). Hwang Y. P.., Kim H. G.., Choi J. H.., Truong Do M.., Tran T. P.., Chun H. K.., Chung Y. C.., Jeong T. C.., Jeong H. G.Mol. Nutr. Food Res. 2013. 57:471–482.
(17). Kim H. S.., Yoon Y. S.., Cho J. W.Korean J. Med. Crop Sci. 2008. 16:231–237.
(18). Kong C. -S.., Lee J. I.., Kim Y. A.., Kim J. -A.., Bak S. S.., Hong J. W.., Park H. Y.., Yea S. S.., Seo Y.Process Biochem. 2012. 47:1073–1078.
(19). Eyjólfsson R.Phytochemistry. 1970. 9:845–851.
(20). Lee K. S.., Kim A. J.., Lee K. Y. J.East Asian Soc. Diet. Life. 2012. 22:521–526.
(21). Kim E. J.., Choi J. Y.., Yu M.., Kim M. Y.., Lee S.., Lee B. -H.Korean J. Food Sci. Technol. 2012. 44:337–342.
(22). Kim S. J.., Lee G.., Moh S. H.., Park J.., Auh C. -K.., Chung Y.., Ryu T. K.., Lee T. -K. J.Korea Acad. Industr. Coop. Soc. 2013. 14(3081–3088):3081–3088.
(23). Singleton V. L.., Rossi J. A.Am. J. Enol. Viticult. 1965. 16:144–158.
(24). Blois M. S.Nature. 1958. 181:1199–1200.
(25). Rosen G. M.., Rauckman E. J.Mol. Pharmacol. 1980. 17:233–238.
(26). Hansen M. B.., Nielsen S. E.., Berg K. J.Immunol. Methods. 1989. 119:203–210.
(27). Engelmann J.., Volk J.., Leyhausen G.., Geurtsen W. J.Biomed. Mater. Res. B. 2005. 75B:272–276.
(28). Kim Y. -M.., de Vera M. E.., Watkins S. C.., Billiar T. R. J.Biol. Chem. 1997. 272:1402–1411.
(29). Moon J. H.., Kim S. Y.., Lee H. G.., Kim S. U.., Lee Y. B.Exp. Mol. Med. 2008. 40:11–18.
(30). Rhee M. H.., Park H. -J.., Cho J. Y. J.Med. Plants Res. 2009. 3:548–555.
(31). Yang E. -J.., Yim E. -Y.., Song G.., Kim G. -O.., Hyun C.-G. Interdiscip. Toxicol. 2009. 2:245–249.
(32). García-Lafuente A.., Guillamón E.., Villares A.., Rostagno M. A.., Martínez J. A.Inflamm. Res. 2009. 58:537–552.
(33). Read M. A.Am. J. Pathol. 1995. 147:235–237.
Go to : 
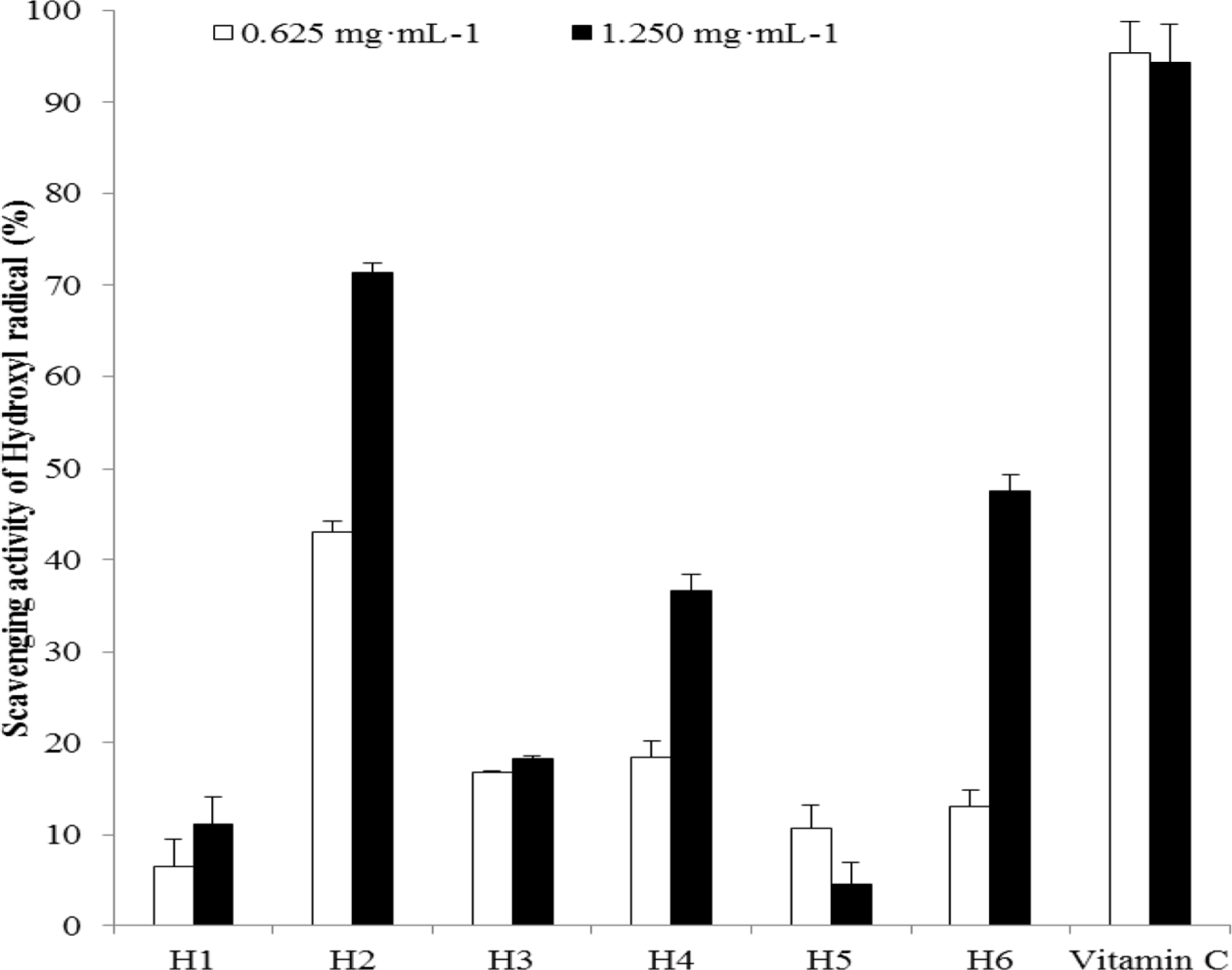 | Fig. 1.Hydroxyl radical scavenging activity of the extracts from six halophytes. Experiment was performed in triplicate and data are represented as mean ± SD. |
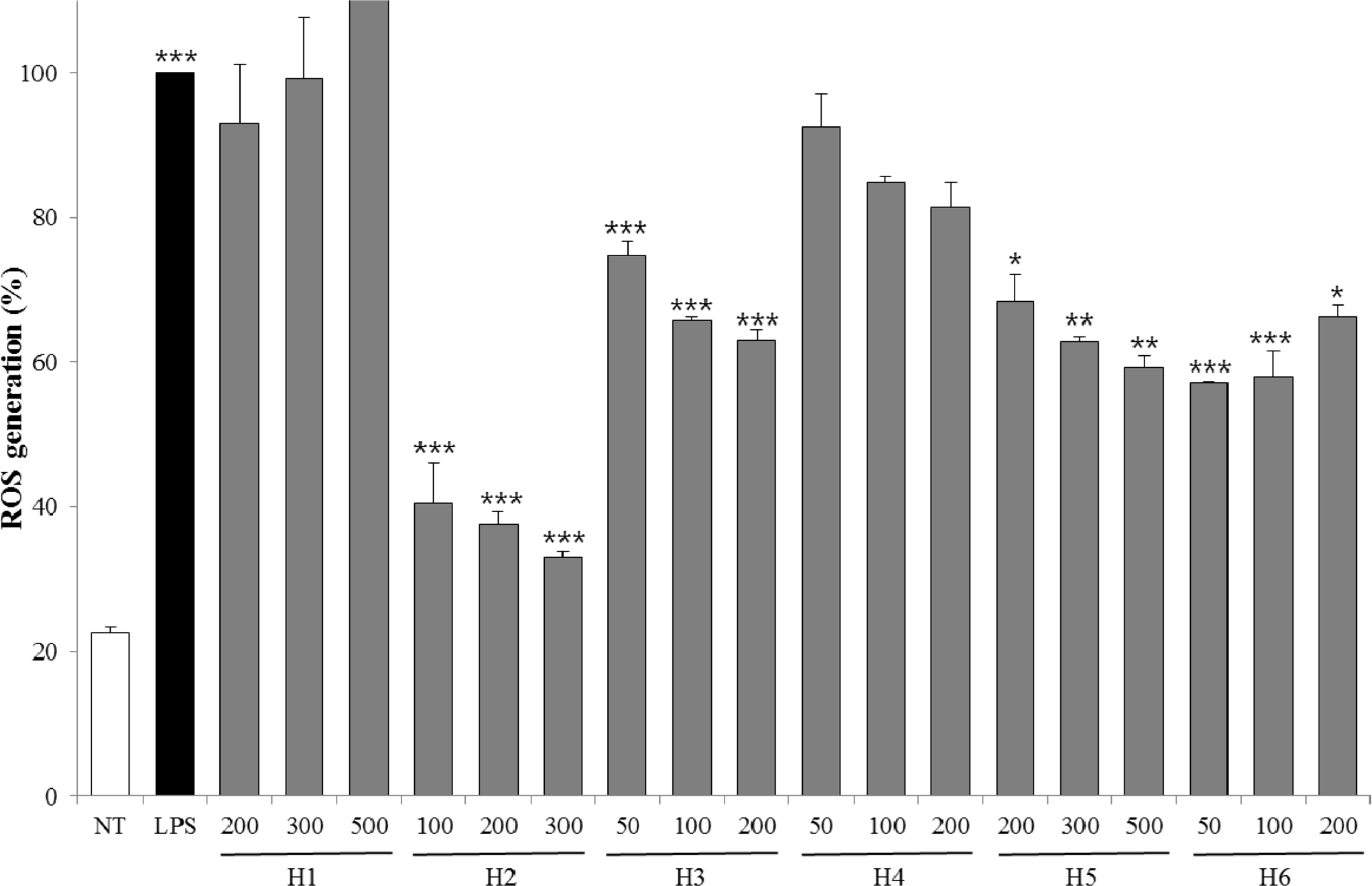 | Fig. 2.ROS generation of extracts from six halophytes. Experiment was performed in triplicate and data are represented as mean ± SD. ∗ p < 0.05; ∗∗ p < 0.01; ∗∗∗ p < 0.001. |
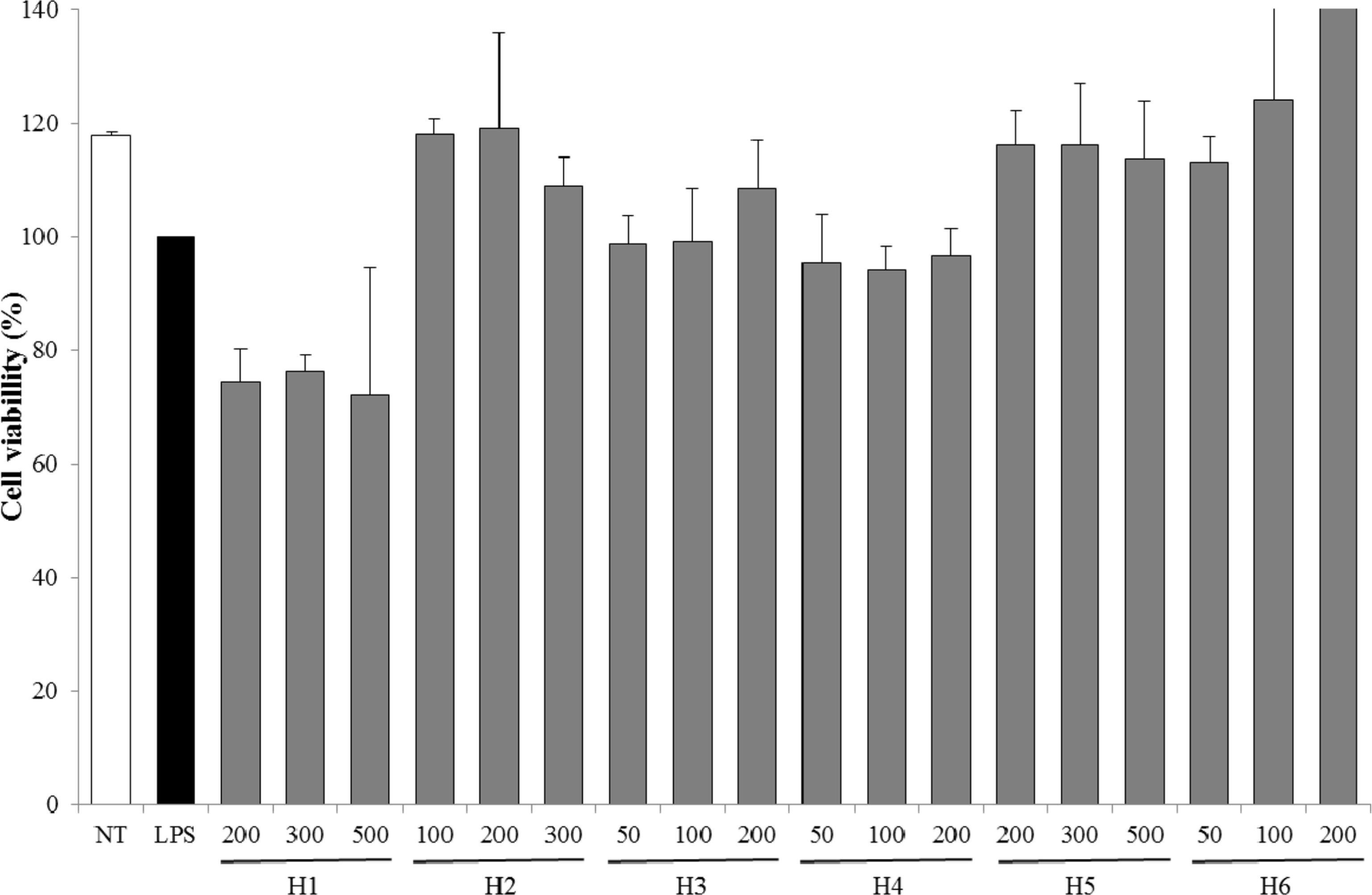 | Fig. 3.Cell viability of extracts from six halophytes. Experiment was performed in triplicate and data are represented as mean ± SD. |
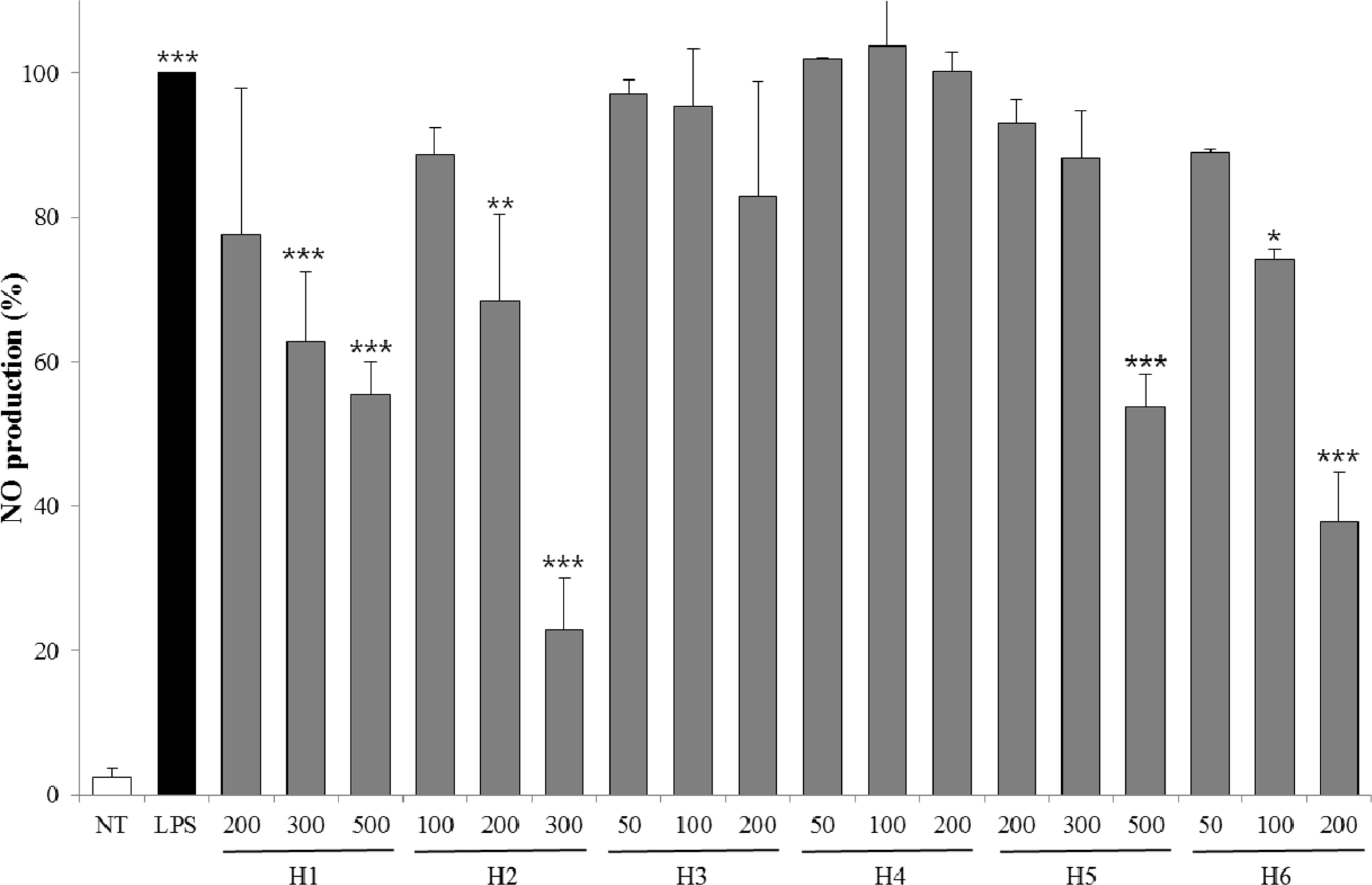 | Fig. 4.Inhibitory effects of NO production for extracts from six halophytes in LPS-RAW 264.7 cells. Experiment was performed in triplicate and data are represented as mean ± SD. ∗ p < 0.05; ∗∗ p < 0.01; ∗∗∗ p < 0.001. |
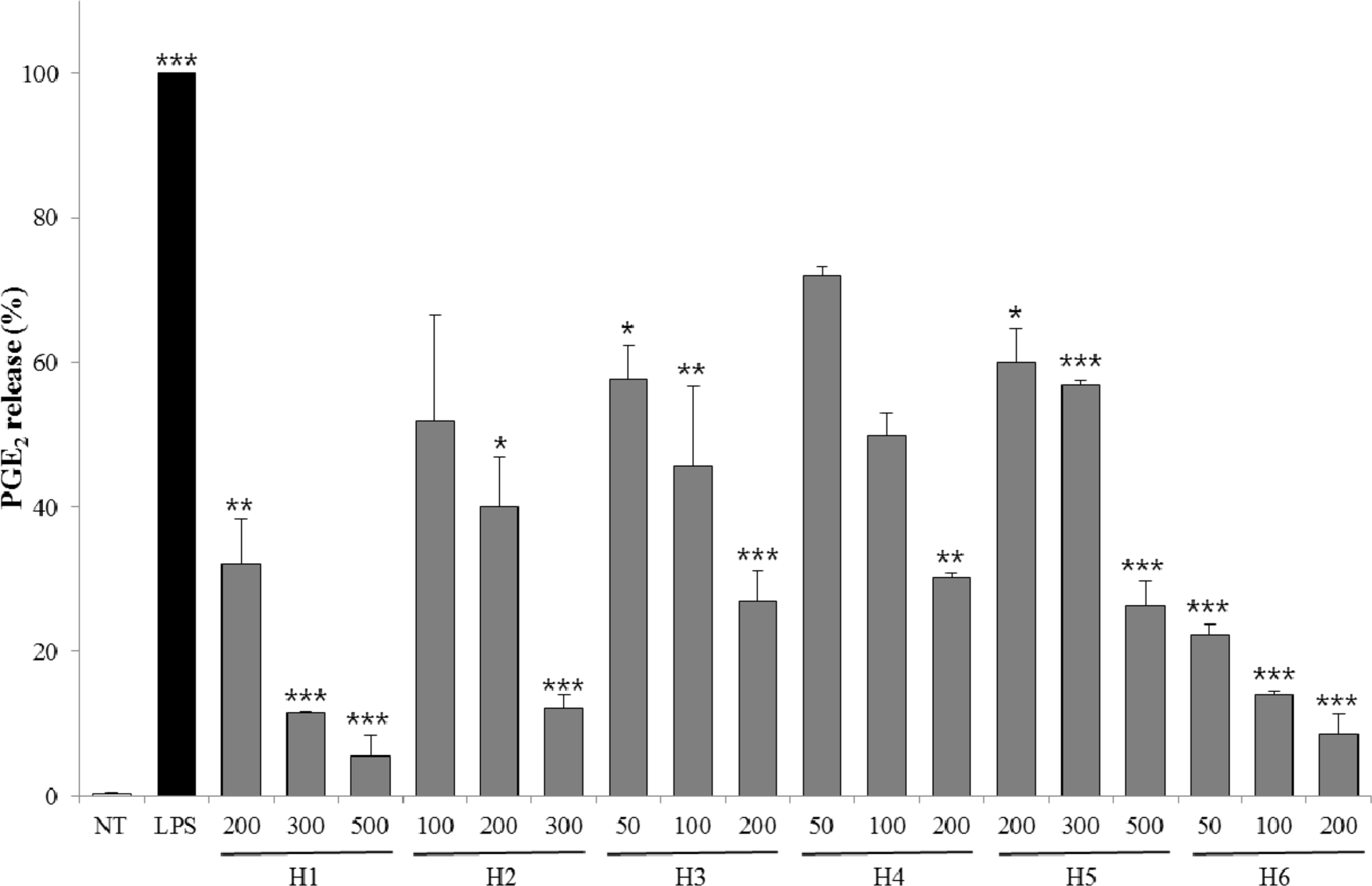 | Fig. 5.Inhibitory effects of PGE2 release for extracts from six halophytes in LPS-RAW 264.7 cells. Experiment was performed in triplicate and data are represented as mean ± SD. ∗ p < 0.05; ∗∗ p < 0.01; ∗∗∗ p < 0.001. |
Table 1.
Total polyphenol content of six halophyte extracts expressed as equivalent of gallic acid (mg of GA/g of extract)
| Species | Total polyphenol content (mg/100 g dry weight) |
|---|---|
| Plumbaginaceae | |
| H1 | 14.27 ± 0.26a |
| Asteraceae | |
| H2 | 59.34 ± 0.32 |
| Chenopodiaceae | |
| H3 | 54.29 ± 0.15 |
| H4 | 52.84 ± 0.46 |
| H6 | 58.71 ± 0.29 |
| Juncaginaceae | |
| H5 | 53.33 ± 0.37 |
Table 2.
DPPH radical scavenging activity of six halophyte extracts expressed as half maximal inhibitory concentration (IC50)
| Species | IC50 (mg/ml) |
|---|---|
| Plumbaginaceae | |
| H1 | 0.020 ± 0.001a |
| Asteraceae | |
| H2 | 0.056 ± 0.001 |
| Chenopodiaceae | |
| H3 | 0.095 ± 0.003 |
| H4 | 0.510 ± 0.003 |
| H6 | 0.061 ± 0.001 |
| Juncaginaceae | |
| H5 | 0.570 ± 0.009 |
| Control | |
| Ascorbic acidb | 0.019 ± 0.004 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download