Abstract
Psoriasis is an auto-immune skin disease, which is characterized by the excessive generation of plaques on the skin with typically a long-lasting red, itchy and scaly symptoms. Imiquimod, which has been used for the treatment of external genital warts, actinic keratosis, and superficial basal cell carcinoma, induced of psoriasis-like skin disorders with skin erythema and thickness in mice. In the present study, we tried to find the bioactive herbal extract against imiquimod-induced psoriasis-like skin disorder in mice. During the searching of the herbal extract with anti-psoriatic effect, the ethanolic extract of Cnidium officinale ameliorated imiquimodinduced psoriasis-like skin disorder in mice. The morphological evaluation, H&E staining and Psoriasis Area and Severity Index (PASI) score showed that ear and back thickness, and erythema induced by imiquimod were significantly reversed after the treatment of the cream of the ethanolic extract of C. officinale. The overexpressed myeloperoxidase (MPO) and keratin 6A levels were decreased by the treatment of C. officinale cream. Also, IFN-γ, c-fos and IκB-α mRNA levels, which are related to the progression of psoriasis, were reduced by C. officinale cream. Thus, the ethanolic extract of C. officinale ameliorated psoriasis-like skin disorder induced by imiquimod and might be the therapeutic agent for psoriasis.
Psoriasis is one of the common skin disorders resulting from the unpredictable malfunction of the auto-immune system.1 Though the symptoms of psoriasis vary depending on the individual situations, the most common symptom is the excessive generation of plaques on the skin, which are typically a long-lasting red, itch and scaly symptom.2 The traditional herb preparations have been used for the treatment of psoriasis without the significant adverse effects.34 For examples, herbal extracts, such as Mahonia aquifolium and Camptotheca acuminata and Aloe vera, have been used as the potential therapeutic agents for the topical management of psoriasis in the clinical test.4
Imiquimod (IMQ) had been approved by US FDA for the treatment of external genital warts, actinic keratosis, and superficial basal cell carcinoma.5 This chemical also is known that it induces of psoriasis-like skin disorders with skin erythema and thickness in animal models.6
Cnidium officinale Makino (Umbelliferae) is one of the traditional herbs native to Korea and has been used for the treatment of female genital inflammatory diseases as a traditional Korean medicine.7 The characteristic metabolites in C. officinale are the volatile phthalide derivatives which exhibit diverse pharmacological activities including of anti-anaemia, anti-fungal, sedative and smooth muscle relaxing.89 Among them, Z-ligustilide is well known as the active phthalide derivative in human keratinocytes through ROS-dependent Nrf2 activation, Nrf/HO-1 upregulation and NF-κB suppression.1011 However, to the best of our knowledge, until now, little has been known about the protective effects of the extract of C. officinale and its components in skin disorder models.
During searching of the attenuating agents of psoriasis symptoms from the plants, we found that C. officinale extract ameliorated IMQ-induced skin disorders in BALB/c mice. Therefore, we could suggest that the preparation consisting of C. officinale might be the therapeutic agent for psoriasis.
Senkyunolide A and Z-ligustilide were obtained from Corescience Inc. (Seoul, Korea). The purities of the standards were more than 98.0%. HPLC system (Agilent Technologies Mfg GmbH&Co.KG, Waldbronn, Germany) consisted of a 1260 quaternary pump, autosampler and multiple wavelength detector carried out the qualitative and quantitative analyses of two compound in C. officinale. Chromatographic separation was performed on a Hector-M C18 column (250 mm × 4.6 mm I.d.; 5 µm). UV detection was set at 290 nm. The mobile phase was the gradient solvent system consisting of solvent A (0.1% formic acid in water) and solvent B (ACN) as follows: linear gradient 70 – 20 – 100% A (0 – 30–34 min) and isocratic 70% A (34 – 40 min). The flow rate was 1.0 mL min−1 and 10 µL of the pulverized 70% ethanolic extract of C. officinale (10 mg/mL) and two standards (500 µg/mL), which were filtered through a 0.45-µm PVDF membrane, were injected using the autosampler for the analyses.
C. officinale extract (1 kg) was extracted in 70% ethanol (1 l) in the Soxhlet apparatus for 3 hr at 65~70 ℃, filtered and freeze-dried to 100 g of powder. It was packaged in the sterilized pouch until the further study. Briefly, for preparing the cream formulation of C. officinale extract (COE), white petrolatum (94.45 g) and sorbitan sesquioleate (0.5 g) were mixed by mild agitation with a magnetic stirring bar (2500 rpm) for 40 min at 80 ℃ and they were cooled at 75 ℃. The pulverized 70% ethanolic extract of C. officinale (0.05 g) and propylene glycol (4 g) were added and mixed by homogenizing at 2500 rpm for 30 min. After adding of propylene glycol (0.5 g), the mixture was blended by stirring at 250 rpm for 10 min at 75 ℃, followed by cooling and stirring at 2000 rpm and 30 ℃ for 10 min. Vehicle cream was prepared by the same procedure except for the addition of the 70% ethanolic extract of C. officinale. COE was stored in the refrigerator (−4 ℃) before the in vivo experiments. A commercially available imiquimod (IMQ) cream (Aldara®, iNova Pharmaceuticals Pty LTD., Chatswood, Australia), clobetasol (CLO) (Dermovate®, Glaxo Operation, UK) and tacrolimus (TAC) (Protopic®, Astellas Pharma Inc., Japan) were gifted from Hankook Korus Pharm, Co., LTD. (Chuncheon, Korea).
BALB/c mice were purchased from Orient Bio Inc. (Sungnam, Korea). Mice were kept under specific pathogen-free conditions and provided with water and food ad libitum. All mice used in the experiments were 8 weeks of age and bred for 7 days in animal care center (temperature 23 ± 2C, humidity 50 ± 5%, 12 h light-dark cycle), Hongcheon Institute of Medicinal Herb (Hongcheon, Korea) before the beginning of the experiments. All experiments were approved by the Institutional Animal Care and Use Committee of Hongcheon Institute of Medicinal Herb (No. 15–04). They were separated into four groups: 1) Vehicle group was the control group treated with control cream; 2) IMQ group was the model group received a daily dose of 62.5mg/day of IMQ mixed with control cream; 3) COE group was the model group treated with only COE; 4) IMQ + CLO group was the model group received CLO cream (17-propionate 0.5 mg/1 g) 6 h later after IMQ treatment; 5) IMQ + TAC group was the model group received TAC cream (tacrolimus 0.1%) 6 h later after IMQ treatment; 6) IMQ + COE group was the model group received COE 6 h later after IMQ treatment. All the experiments were treated for every 2 days, during 6 days.
The samples were rubbed 1 day after the removal of ear and back hairs for 0, 2, 4 and 6 days. The ear and back thickness were measured using Digimatic Thickness Gage (model no. 547-500S, Mitutoyo, Japan) using the hair removal products (Veet®, Oxy Reckitt Benckiser, Slough, UK). The erythema severity was expressed using Dermacatch® (Colorix, Swiss).
The severity of psoriasis was measured by the Psoriasis Area and Severity Index (PASI). Briefly, the ear and back thickness, and erythema were scored independently and calculated according to PASI index (http://pasi.corti.li, ver. 1.7.1).
Ear and back tissues were washed with PBS and fixed in 10% neutral buffered formalin (NBF) for 24 hours at room temperature. The tissues were dehydrated by gradually soaking them in alcohol and xylene and then embedded in paraffin. The paraffin-embedded specimens were cut into 5 µm sections, stained with hematoxylin and eosin (H&E) and observed with a digital light microscope (Axio Scan. Z1, Zeiss, Germany).
For evaluation of the changes of myeloperoxidase (MPO) and keratin 6A, sections were deparaffinized and incubated with mouse anti-MPO (Abcam, Cambridge, UK) and mouse anti-keratin 6A (Abcam, Cambridge, UK) in humidified chamber at 4 ℃ for 24 hours, followed by biotinylated goat anti-mouse IgG-B (SantaCruz Biotechnology, Santa Cruz, USA) at 4 ℃ for 24 hours. Immunostaining was performed by tris-HCl (pH 7.4) buffered with 0.05% 3,3′-diaminobenzidin (Sigma-Aldrich, St. Louis, USA) and 0.01% HCl 1 hour after the incubation with an avidin-biotin-HRP complex (Dako, Carpinteria, USA), and observed with a digital light microscope (Axio Scan. Z1, Zeiss, Germany).
Total mRNA was extracted from 50~100 mg of back skin with 1 ml of Trizol reagent (Invitrogen, USA) by homogenization. The mRNA was transcribed to cDNA with cDNA reverse transcription kit (Qiagen, Japan). IFN-γ, c-fos and IκB-α mRNA levels were measured by a SYBR Green (Applied Biosystems, USA) and 7500 Real-time Thermal Cycler (Applied Biosystems, USA). The specific primers were: IFN-γ: forward primer, 5′-TCA AGT GGC ATA GAT GTG GAA GAA-3′, reverse primer, 5′-TGG CTC TGC AGG ATT TTC ATG-3′; c-fos: forward primer, 5′-CCT TCG GAT TCT CCG TTT CTC-3′, reverse primer, 5′-TGG TGA AGA CCG TGT CAG GA-3′; IκB-α: forward primer, 5′-TGT CTG CAC CTA GCC TCT ATC CA-3′, reverse primer, 5′-ATC AGC ACC CAA AGT CAC CAA-3′; β-actin: forward primer, 5′-GGC TGT ATT CCC CTC CAT CG-3′, reverse primer, 5′-CCA GTT GGT AAC AAT GCC ATG T-3′. The results were analyzed by One step system software ver. 2.1 (Applied Biosystems, USA).
Data were expressed as the mean ± SD unless indicated otherwise. One-way ANOVA with the Tukey-HSD post hoc test was performed to compare the differences between two groups using GraphPad Prism 6.0 version (GraphPad Software, Inc., San Diego, CA, USA). A value of P < 0.05 was considered significant at the 95% confidence level (*p < 0.05).
The LC chromatogram of the 70% ethanolic extract of C. officinale used in the present study was shown in Fig. 1, and senkyunolide A and Z-ligutilide were detected as the major components of C. officinale.7 The thickness of back and ear was unequivocally increased 2–3 days after IMQ application (62.5 mg/day) with the characteristic changes of psoriasis lesions, such as hyperkeratosis and desquamation compared to the positive controls, clobetasol and tacrolimus (Fig. 2a).12 COE ameliorated the thickness and scaling of back and ear, while back and ear erythema was not changed compared to IMQ group (Fig. 2a). This result related to erythema may be due to the colorization of COE on back and ear. It was consistent of the results of the independent scores in a representative experiment (Fig. 2b). After 6 days, the relative changes of the thickness of back and ear, erythema and scaling were scored together on a scale from 0 to 4 and applied to the PASI index (Fig. 2b). The PASI score of only IMQ-induced group (PASI score = 8.87) was significantly increased decreased compared to vehicle group (0.15). However, it was reversed psoriasis-like symptoms after the treatment of COE (5.56).
The effect of COE on the psoriasislike symptom induced by IMQ symptom was confirmed by the histological (H&E staining) and immunohistochemical analyses on back and ear. The treatment of IMQ locally induced and exacerbated the symptoms of psoriasislike skin lesions, such as epidermal acanthosis, the presence of nuclei in the stratum corneum (parakeratosis) in the back and ear skin, while COE significantly ameliorated these symptoms (Fig. 3a and 3b). Keratin 6A which is one of the psoriasis-typical markers was overexpressed in the IMQ-treated group (Fig. 4a and 4b).13 In the COE-treated group, the abnormal proliferation induced by IMQ was significantly ameliorated. Also, the overexpressed MPO level, which is another marker for the psoriasis-like skin lesion, was reversed after the treatment of COE (Fig. 4c and 4d).
We next examined the protective mechanism of COE against psoriasis-like lesion. It has been previously reported that IMQ has its immunomodulatory activity via the induction of the release of IFN-γ.14 Though IMQ may be effective in the infected disease, such as leishmaniasis, but the prolonged exposure may cause psoriasis-like symptoms. In the present study, IMQ induced the expression of IFN-γ, c-fos and IκB-α, which are highly secreted from immune cells, such as dendritic cells, macrophages, and T cells, and related to the stimulation of the keratinocytes proliferation.1516 However, COE significantly reduced the expression of IFN-γ, c-fos and IκB-α (Fig. 5).
Psoriasis is an auto-immune skin disorder characterized by the hyper-proliferation of keratocytes. The pathogenic factors of psoriasis such as genetic background, viral infection, metabolic syndromes, oxidative stress and immune responses have been reported.17 However, despite extensive studies on the pathogenesis of psoriasis, the detailed mechanism of psoriasis remains elusive.
The extracts from natural products have been studied for the development of the therapeutic candidates for psoriasis in folk medicine.18192021 The multiple constituents in the extracts can produce a wide range of preventive and therapeutic effects related to psoriasis.22 It has been reported that C. officinale extract has the protective effects against ultraviolet B (UVB)-induced DNA damage, and H2O2-induced DNA and cell damage in the human skin fibroblast cell.2324 Though it was reported that Z-ligustilide, its bioactive metabolites of C. officinale, has the antiinflammatory effect by suppressing the NF-κB pathway, its extract has not been reported it has the protective effect on skin disorder.
In the present study, IMQ could be used as the inducer of psoriasis-like skin inflammation in BALB/c mice via the IL-23/IL-17 axis and for the evaluation of new therapies in psoriasis.6 The IMQ-treated group showed obvious psoriasis-like symptoms compared with the control group. The histological analysis of the skin in the IMQ-treated group revealed epidermal thickening and erythema, and the overexpression of keratin 6A and MPO, while COE reversed the skin deterioration by the treatment of IMQ. Furthermore, COE significant decreased highly proliferated keratin 6A and MPO in the IMQtreated mice, which are the pathological markers in psoriasis.1325 We also confirmed that COE reduced the inflammatory signals, IFN-γ, c-fos and IκB-α, significantly associated with excessive hyperproliferation of keratinocytes, which are one of the pathologic conditions of psoriasis.1426
Though the mechanism of action of COE in psoriasis, such as the changes of protein levels related to psoriasis-like skin inflammation, needs further research, it was suggested that COE regulated IMQ-induced psoriasis-like symptoms and also reduced the inflammatory signals including of IFN-γ, c-fos and IκB-α. Consequently, these results might be expected that the ethanolic extract of C. officinale can be the therapeutic agents for psoriasis.
Figures and Tables
 | Fig. 1HPLC chromatogram of Cnidium rhizome and two chemical markers obtained at 290 nm. (a): the water extract of Cnidium rhizome (10 mg/mL); (b): Z-ligustilide; (c): senkyunolide A. |
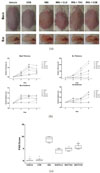 | Fig. 2COE (the cream formulation of the 70% ethanolic extract of C. officinale) ameliorates IMQ-induced psoriasis-like lesion on back skin and ear of BALB/c mice. The experiments were performed as described in material and methods. a, mice were treated daily with vehicle cream, COE, IMQ cream, IMQ + CLO cream, IMQ + TAC cream and IMQ + COE on the shaved back skin and ear. b, the cumulative scores (%) of thickness and erythema of back skin and ear for 6 days, respectively, relative to 0 day. c, the PASI scores were measured as the sum of the ear and back thickness, and erythema according to PASI index (http://pasi.corti.li, ver 1.7.1) after the final administration. Symbols indicate mean score ± SEM of six groups, n = 6/group. *p < 0.05 indicates a statistically significant difference from the IMQ group. |
 | Fig. 3COE alters keratinocyte proliferation and differentiation. H&E staining (× 100) of back skin (a) and ear (b). Mice were treated daily with vehicle cream, COE, IMQ cream, IMQ + CLO cream, IMQ + TAC cream and IMQ + COE for 6 days. K: keratin of stratum corneum, E: epidermis, HF: hair follicles, D: dermis and SC: subcutis. |
 | Fig. 4COE alters the expression of keratin 6A of back skin (a) and ear (b), and MPO of back skin (c) and ear (d) (IHC, × 100). Mice were treated daily with vehicle cream, COE, IMQ cream, IMQ + CLO cream, IMQ + TAC cream and IMQ + COE for 6 days. |
 | Fig. 5COE reduced the expression of IFN-γ, c-fos and IκB-α induced by IMQ. Mice were treated daily with vehicle cream, COE, IMQ cream and IMQ + COE for 6 days. RNA was extracted from back skin after sacrifice. The expression of IFN-γ, c-fos and IκB-α was determined by RT-PCR and their levels were represented relative to β-actin levels in an individual mouse (n = 6). |
Acknowledgements
This research was supported by the Ministry of Trade, Industry & Energy (MOTIE), Korea Institute for Advancement of Technology (KIAT) through the Encouragement Program for The Industries of Economic Cooperation Region.
References
1. Sakkas LI, Bogdanos DP. Autoimmun Rev. 2017; 16:10–15.
2. Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, Lebwohl M, Koo JYM, Elmets CA, Korman NJ, Beutner KR, Bhushan R. J Am Acad Dermatol. 2008; 58:826–850.
3. Zhang CS, Yu JJ, Parker S, Zhang AL, May B, Lu C, Xue CC. Int J Dermatol. 2014; 53:1305–1318.
4. Deng S, May BH, Zhang AL, Lu C, Xue CCL. Br J Dermatol. 2013; 169:769–782.
5. OuYang Q, Pan Y, Luo H, Xuan C, Liu J, Liu J. Int Immunopharmacol. 2016; 39:369–376.
6. van der Fits L, Mourits S, Voerman JSA, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E. J Immunol. 2009; 182:5836–5845.
8. Bae KE, Choi YW, Kim ST, Kim YK. Molecules. 2011; 16:8833–8847.
9. Ramalingam M, Park YK. Pharmacogn Mag. 2010; 6:323–330.
10. Wu Z, Uchi H, orino-Koga S, Nakamura-Satomura A, Kita K, Shi W, Furue M. Exp Dermatol. 2014; 23:260–265.
11. Wu Z, Uchi H, Morino-Koga S, Shi W, Furue M. Exp Dermatol. 2015; 24:703–708.
12. Vincent N, Ramya DD, Vedha HB. Dermatol Reports. 2014; 6:5451.
13. Mommers JM, van Rossum MM, van Erp PEJ, Van de Kerkhof PCM. Dermatology. 2000; 201:15–20.
15. Palfreeman AC, McNamee KE, McCann FE. Drug Des Devel Ther. 2013; 7:201–210.
16. Nestle FO, Kaplan DH, Barker J. N Engl J Med. 2009; 361:496–509.
17. Hou R, Li J, Niu X, Liu R, Chang W, Zhao X, Wang Q, Li X, Yin G, Zhang K. J Dermatol Sci. 2017; 86:181–186.
18. Han R, Rostami-Yazdi M, Gerdes S, Mrowietz U. Br J Clin Pharmacol. 2012; 74:424–436.
19. Bello I, Shehu MW, Musa M, Asmawi MZ, Mahmud R. J Ethnopharmacol. 2016; 189:253–276.
20. Choudhry SZ, Bhatia N, Ceilley R, Hougeir F, Lieberman R, Hamzavi I, Lim HW. J Drugs Dermatol. 2014; 13:148–153.
21. Lee YM, Kim NH, Lee MR. Nat Prod Sci. 2015; 21:122–127.
22. Belcaro G, Maquart FX, Scoccianti M, Dugall M, Hosoi M, Cesarone MR, Luzzi R, Cornelli U, Ledda A, Feragalli B. Panminerva Med. 2011; 53:105–118.
23. Jeong JB, Ju SY, Park JH, Lee JR, Yun KW, Kwon ST, Lim JH, Chung GY, Jeong HJ. Cancer Epidemiol. 2009; 33:41–46.
24. Jeong JB, Park JH, Lee HK, Ju SY, Hong SC, Lee JR, Chung GY, Lim JH, Jeong HJ. Food Chem Toxicol. 2009; 47:525–529.
25. Cao LY, Soler DC, Debanne SM, Grozdev I, Rodriguez ME, Feig RL, Carman TL, Gilkeson RC, Orringer CE, Kern EF, McCormick TS, Cooper KD, Korman NJ. Am J Transl Res. 2013; 6:16–27.
26. Freedberg IM, Tomic-Canic M, Komine M, Blumenberg M. J Invest Dermatol. 2001; 116:633–640.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download