Abstract
Estragole is a naturally occurring phenylpropanoid obtained from essential oils found in a broad diversity of plants. Although the phenylpropanoids show many biological activities, clear regulation of the inflammatory signaling pathways has not yet been determined. Here, we scrutinized the anti-inflammatory effect of estragole. The anti-inflammatory effect of estragole was determined through the inhibitory mechanisms of inducible nitric oxide synthase (iNOS), cyclooxygenase (COX-2), nuclear factor kappa B (NF-κB), and mitogen-activated protein kinases (MAPK) pathways and the activation of nuclear factor erythroid 2-related factor 2 (Nrf-2)/heme oxygenase (HO)-1 pathways in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells. Estragole significantly inhibited NO production, iNOS and COX-2 expression as well as LPS-induced NF-κB and MAPK activation. Furthermore, estragole suppressed LPS-induced intracellular ROS production but up-regulated the stress response gene HO-1 via the activation of transcription factor Nrf-2. These findings demonstrate that estragole inhibits the LPS-induced expression of inflammatory mediators via the down-regulation of iNOS, COX-2, NF-κB, and MAPK pathways, as well as the up-regulation of the Nrf-2/HO-1 pathway, indicating that this phenylpropanoid has potential therapeutic and preventive applications in various inflammatory diseases.
Go to : 
References
(1). Joung E. -J.., Lee B.., Gwon W. -G.., Shin T.., Jung B. -M.., Yoon N. -Y.., Choi J. S.., Oh C. -W.., Kim H.-R. Int. Immunopharmacol. 2005. 29:693–700.
(2). Laroux F. S.., Pavlick K. P.., Hines I. N.., Kawachi S.., Harada H.., Bharwani S.., Hoffman J. M.., Grisham M. B.Acta Physiol. Scand. 2001. 173:113–118.
(3). Giuliani C.., Napolitano G.., Bucci I.., Montani V.., Monaco F.Clin. Ter. 2001. 152:249–253.
(4). May M. J.., Ghosh S.Immunol. Today. 1998. 19:80–88.
(5). Tak P. P.., Firestein G. S. J.Clin. Invest. 2001. 107:7–11.
(6). Cargnello M.., Roux P. P.Microbiol. Mol. Biol. Rev. 2011. 75:50–83.
(7). Chen H. -G.., Xie K. -L.., Han H. -Z.., Wang W. -N.., Liu D. -Q.., Wang G. -L.., Yu Y. -H.Int. J. Surg. 2013. 11:1060–1066.
(8). Taha R.., Blaise G.Funct. Food Health Dis. 2014. 4:510–523.
(9). Bakkali F.., Averbeck S.., Averbeck D.., Idaomar M.Food Chem. Toxicol. 2008. 46:446–475.
(10). Choi J. S.., Song B. M.., Park H. J.Kor. J. Pharmacogn. 2016. 47:192–196.
(11). Donati M.., Mondin A.., Chen Z.., Miranda F. M.., do Nascimento B. B. Jr.., Schirato G.., Pastore P.., Froldi G.Nat. Prod. Res. 2015. 29:939–946.
(12). Friedman M.., Henika P. R.., Mandrell R. E. J.Food Prot. 2002. 65:1545–1560.
(13). Cosentino R. M.., Norte M. C.., Lazarini C. A.Phytother. Res. 2004. 18:921–924.
(14). Albuquerque A. A.., Sorenson A. L.., Leal-Cardoso J. H. J.Ethnopharmacol. 1995. 49:41–49.
(15). Ponte E. L.., Sousa P. L.., Rocha M. V.., Soares P. M.., Coelho-de-Souza A. N.., Leal-Cardoso J. H.., Assreuy A. M.Pharmacol. Rep. 2012. 64:984–990.
(16). Rodrigues L. B.., Oliveira Brito Pereira Bezerra Martins A.., Cesário F. R.., Ferreira e Castro F.., de Albuquerque T. R.., Martins Fernandes M. N.., Fernandes da Silva B. A.., Quintans Júnior L. J.., da Costa J. G.., Melo Coutinho H. D.., Barbosa R.., Alencar de Menezes I. R.Chem. Biol. Interact. 2016. 257:14–25.
(17). Keiser K.., Johnson C. C.., Tipton D. A. J.Endod. 2000. 26:288–291.
(18). Meda L.., Cassatella M. A.., Szendrei G. I.., Otvos L. Jr.., Baron P.., Villalba M.., Ferrari D.., Rossi F.Nature. 1995. 374:647–650.
(19). Dandona P.., Chaudhuri A.., Dhindsa S.Diabetes Care. 2010. 33:1686–1687.
(20). Marks-Konczalik J.., Chu S. C.., Moss J. J.Biol. Chem. 1998. 273:22201–22208.
(21). Islam M. N.., Choi R. J.., Jin S. E.., Kim Y. S.., Ahn B. R.., Zhao D.., Jung H. A.., Choi J. S. J.Ethnopharmacol. 2012. 144:175–181.
(22). Chen J. -J.., Huang W. -C.., Chen C.-C. Mol. Biol. Cell. 2005. 16:5579–5591.
(23). Rajapakse N.., Kim M. M.., Mendis E.., Kim S. K.Immunology. 2008. 123:348–357.
(24). Kaminska B.Biochem. Biophys. Acta. 2005. 1754:253–262.
(25). Xia Z.., Dickens M.., Raingeaud J.., Davis R. J.., Greenberg M. E.Science. 1995. 24:1326–1331.
(26). Hancock J. T.., Desikan R.., Neill S. J.Biochem. Soc. Trans. 2001. 29:345–350.
(27). Choi S. Y.., Hwang J. H.., Ko H. C.., Park J. G.., Kim S. J. J.Ethnopharmacol. 2007. 113:149–155.
(28). Siomek A.Acta Biochem. Pol. 2012. 59:323–331.
(29). Ryan K. A.., Simth M. F. Jr.., Sanders M. K.., Ernst P. B.Infect. Immun. 2004. 72:2123–2130.
(30). Kim J. -H.., Choo Y. -Y.., Tae N.., Min B. -S.., Lee J.-H. Int. Immunopharmacol. 2014. 22:420–426.
(31). Lee I. -S.., Lim J.., Gal J.., Kang J. -C.., Kim H. -J.., Kang B. -Y.., Choi H.-J. Neurochem. Int. 2011. 58:153–160.
(32). Paine A.., Eiz-Vesper B.., Blasczyk R.., Immenschuh S.Biochem. Pharmacol. 2010. 80:1895–1903.
(33). Lee M. -Y.., Lee J. -A.., Seo C.-S.., Ha H.., Lee H.., Son J. -K.., Shin H.-K. Food Chem. Toxicol. 2011. 49:1047–1055.
(34). Tsoyi K.., Lee T. Y.., Lee Y. S.., Kim H. J.., Seo H. G.., Lee J. H.., Chang K. C.Mol. Pharmacol. 2009. 76:173–182.
Go to : 
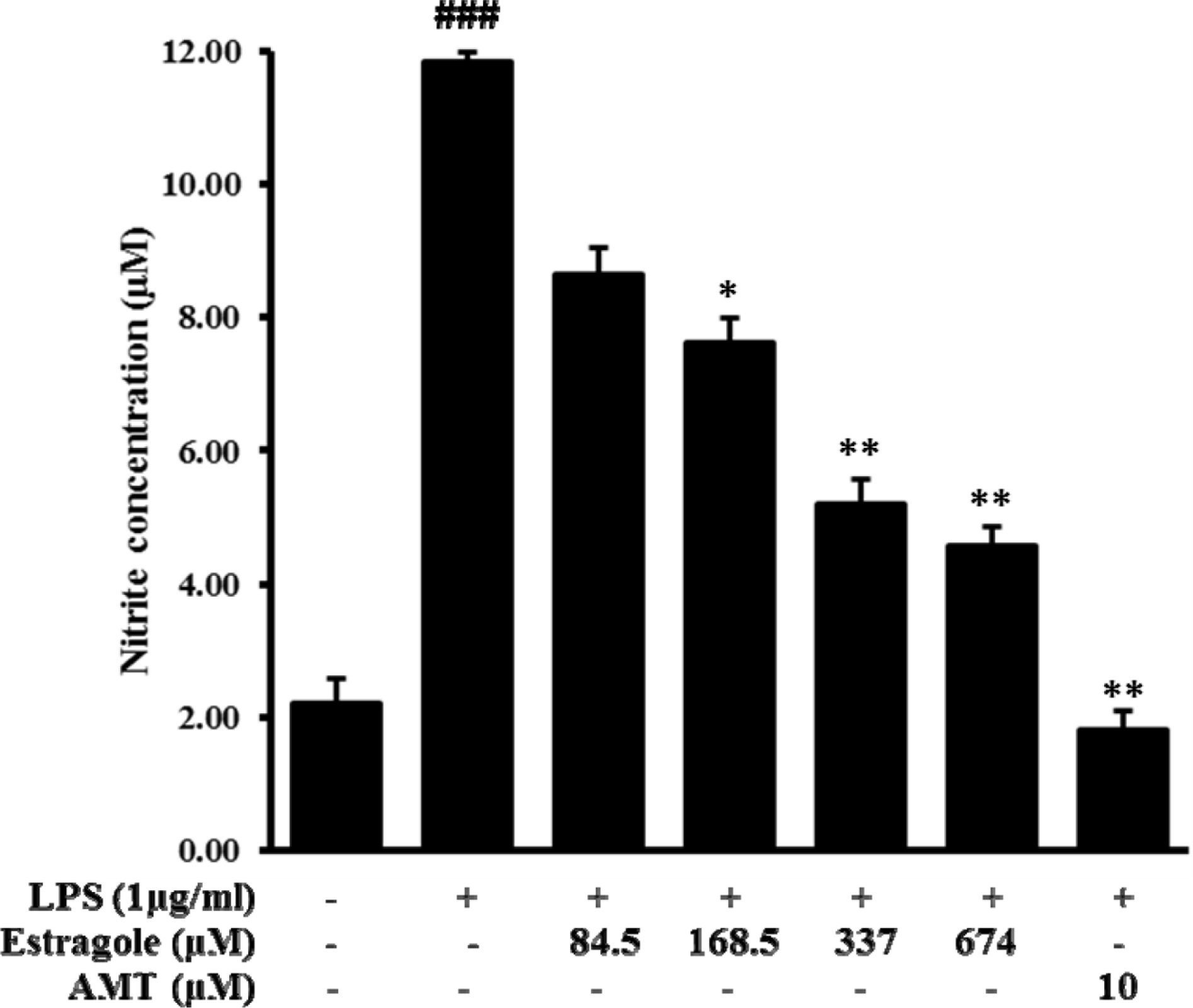 | Fig. 1.Inhibitory effect of estragole on the production of nitric oxide (NO) in LPS-stimulated RAW264.7 cells. Cells were pretreated with different concentrations of estragole for 2 h and stimulated with LPS (1 μg/mL) for 24 h. NO production was measured by Griess reaction. Data are presented as the mean ± standard deviation of three independent experiments.###p < 0.001 indicates significant differences from the control group. ∗p <0.05, ∗∗p <0.01, and ∗∗∗p <0.001 indicate significant differences from the LPS-treated group. |
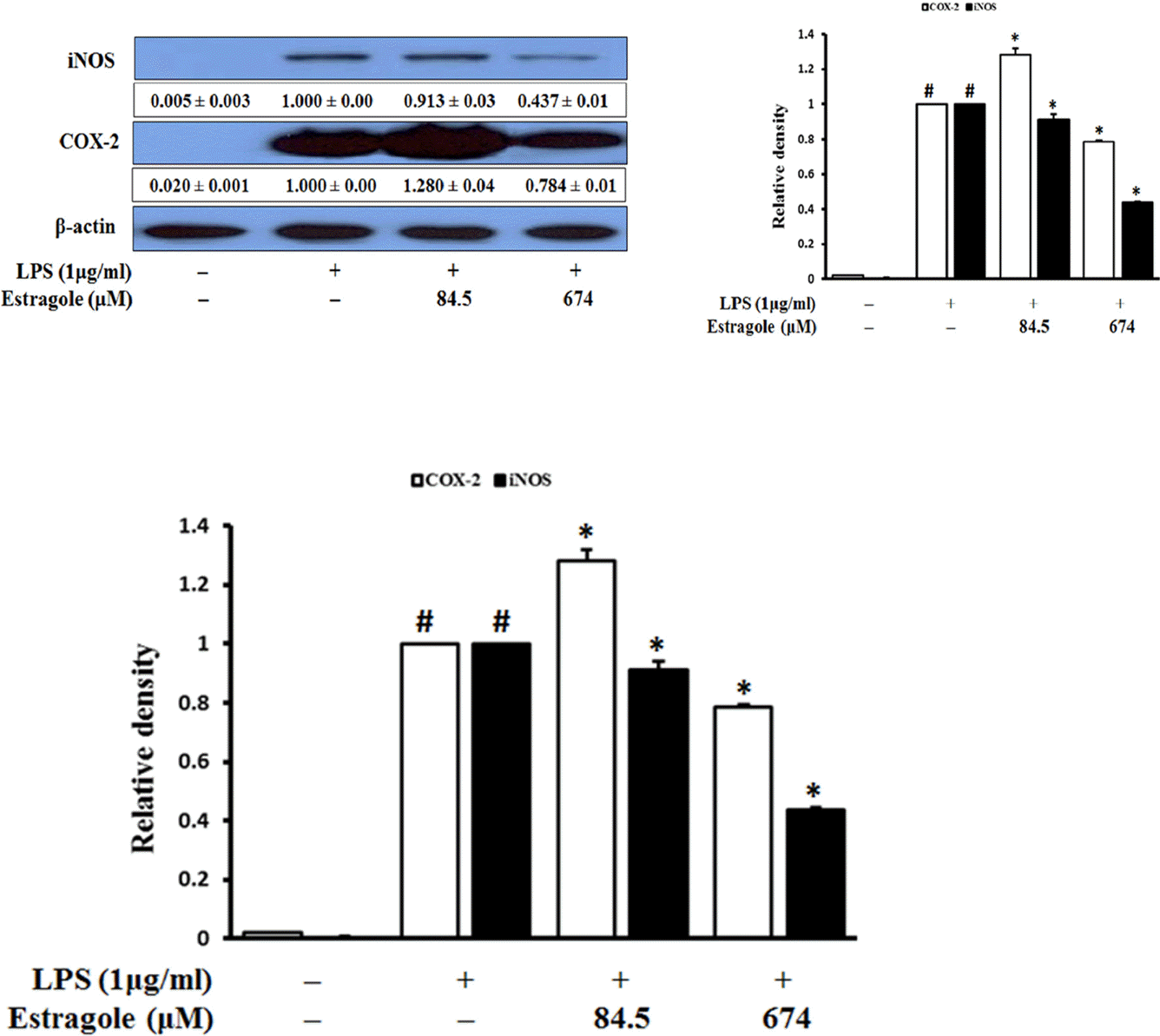 | Fig. 2.Inhibitory effect of estragole on the expression of iNOS and COX-2 in LPS-stimulated RAW264.7 cells. Cells were pretreated with the indicated concentration of estragole for 2 h and stimulated with LPS (1 μg/mL) for 18 h. Data was detected by Western blot analysis with the designated antibodies. β-Actin was used as an internal control. The results presented are representative of three independent experiments. #p < 0.05 indicates significant differences from the control group. ∗p < 0.05 indicates significant differences from the LPS-treated group. |
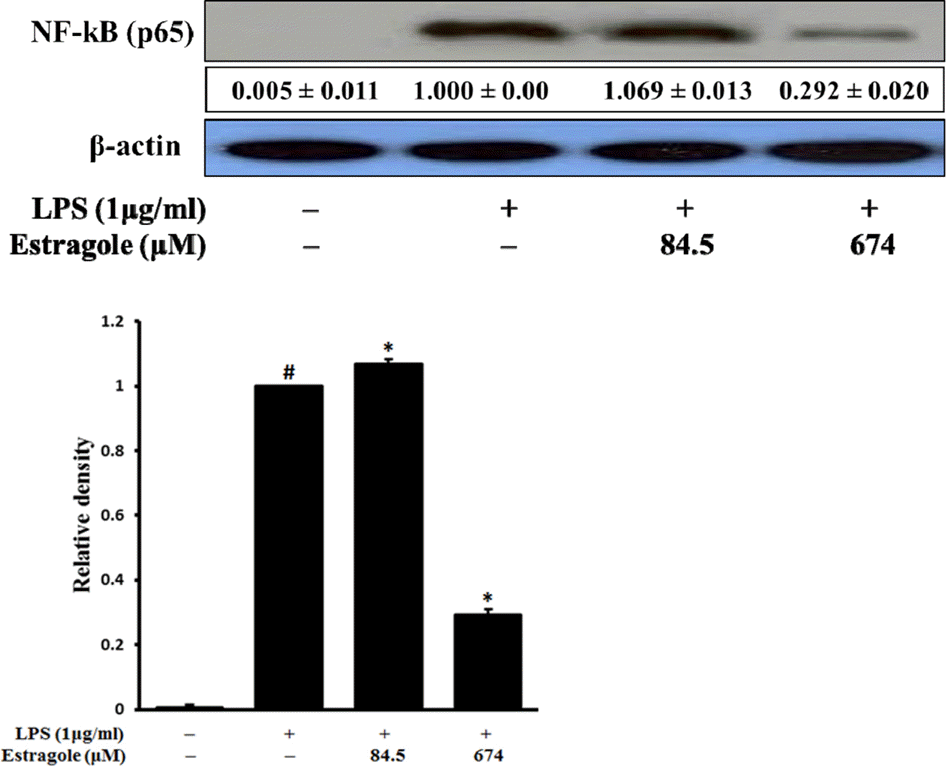 | Fig. 3.Inhibitory effect of estragole on the expression of NF-κB (total protein) in LPS-stimulated RAW264.7 cells. Cells were pretreated with the indicated concentrations of estragole for 2 h and stimulated with LPS (1 μg/mL) for 18 h. Data was detected by Western blot analysis with the designated antibodies. β-Actin was used as an internal control. The results presented are representative of three independent experiments. #p <0.05 indicates significant differences from the control group. ∗p < 0.05 indicates significant differences from the LPS-treated group. |
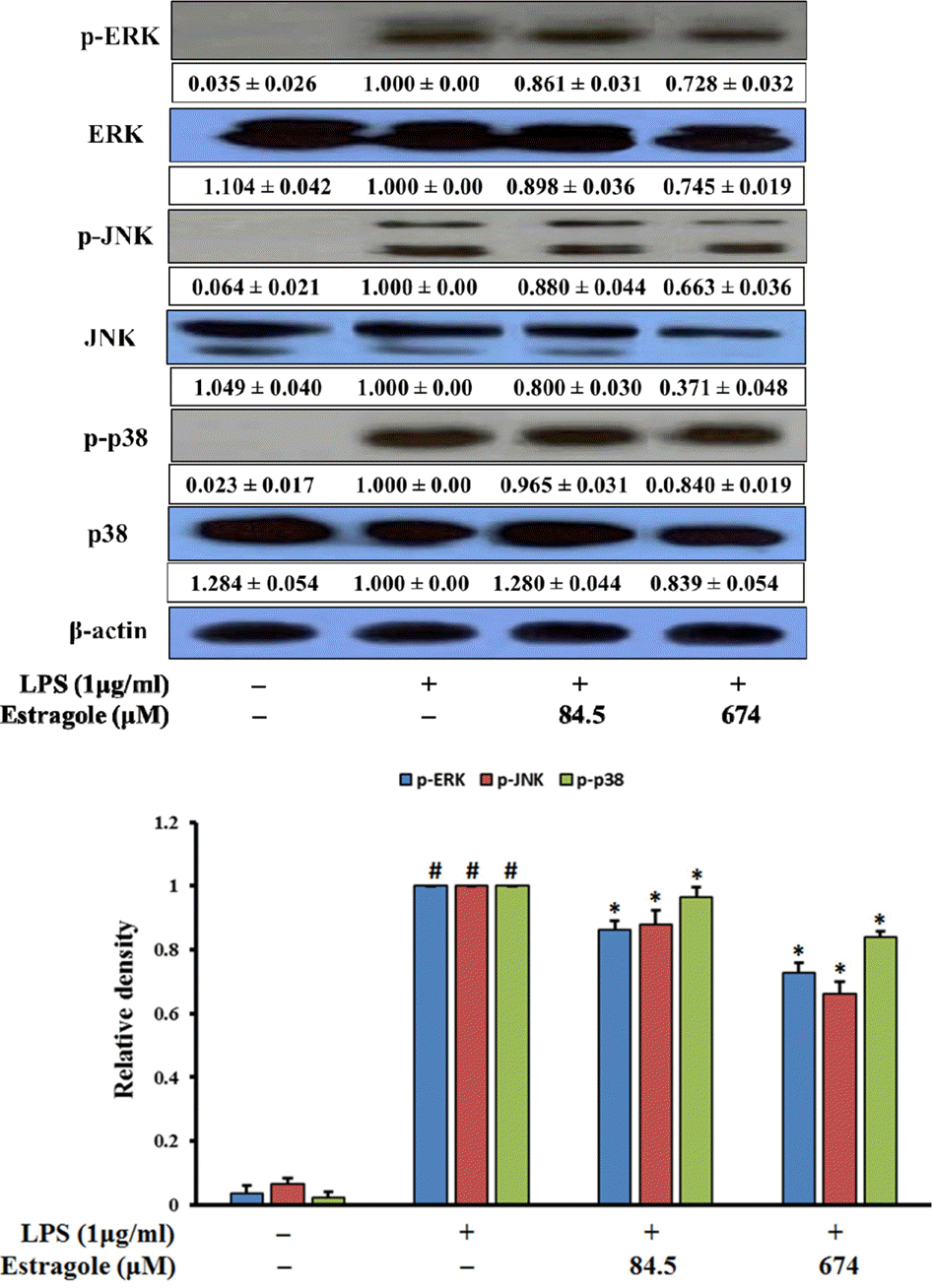 | Fig. 4.Inhibitory effect of estragole on the expression of MAPKs in LPS-stimulated RAW264.7 cells. Cells were pretreated with the indicated concentrations of estragole for 2 h and stimulated with LPS (1 μg/mL) for 18 h. Data was detected by Western blot analysis with the designated antibodies. β-Actin was used as an internal control. The results presented are representative of three independent experiments. #p <0.05 indicates significant differences from the control group. ∗p < 0.05 indicates significant differences from the LPS-treated group. |
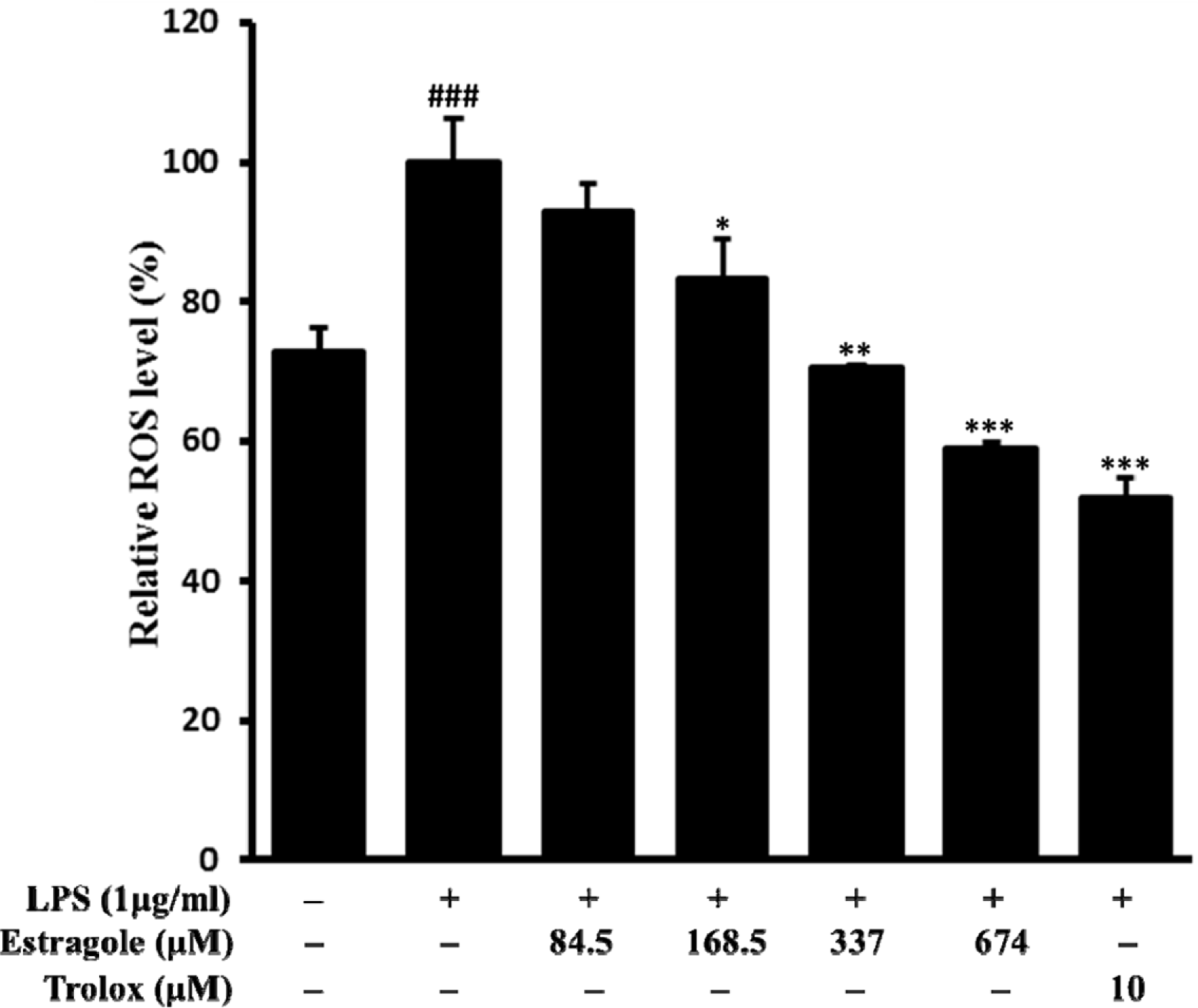 | Fig. 5.Inhibitory effect of estragole on the production of ROS in LPS-stimulated RAW264.7 cells. Cells pretreated with different concentrations of estragole for 2 h were stimulated with LPS (1 μg/mL) for 24 h. ROS levels were measured by DCFH-DA with fluorescent analysis. Data are presented as the mean ± standard deviation of three independent experiments. ###p < 0.01 indicates significant differences from the control group. ∗p < 0.05, ∗∗p <0.01, and ∗∗∗p <0.001 indicate significant differences from the LPS-treated group. |
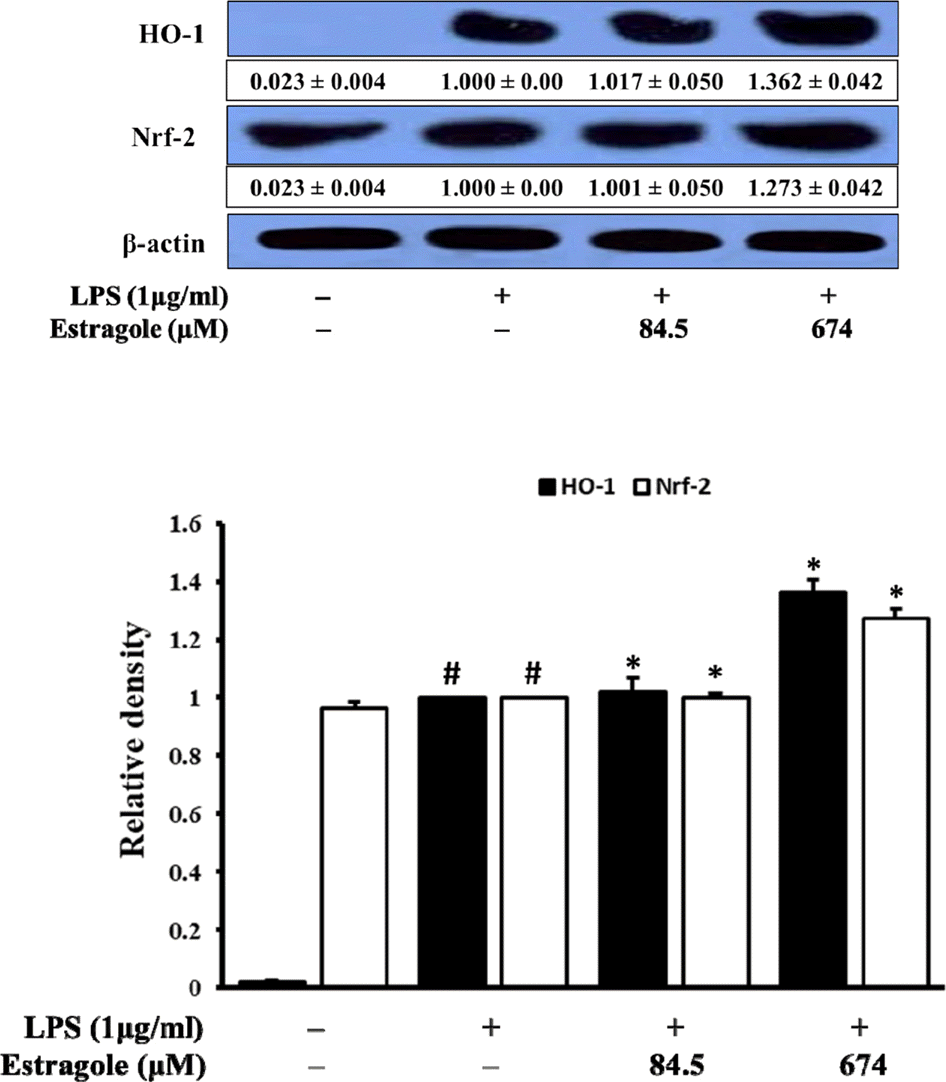 | Fig. 6.Inhibitory effect of estragole on the expression of HO-1 and Nrf-2 in LPS-stimulated RAW264.7 cells. Cells were pretreated with the indicated concentration of estragole for 2 h and stimulated with LPS (1 μg/mL) for 18 h. Data was detected by Western blot analysis with the designated antibodies. β-Actin was used as an internal control. The results presented were representative of three independent experiments. #p < 0.05 indicates significant differences from the control group. ∗p < 0.05 indicates significant differences from the LPS-treated group. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download