Abstract
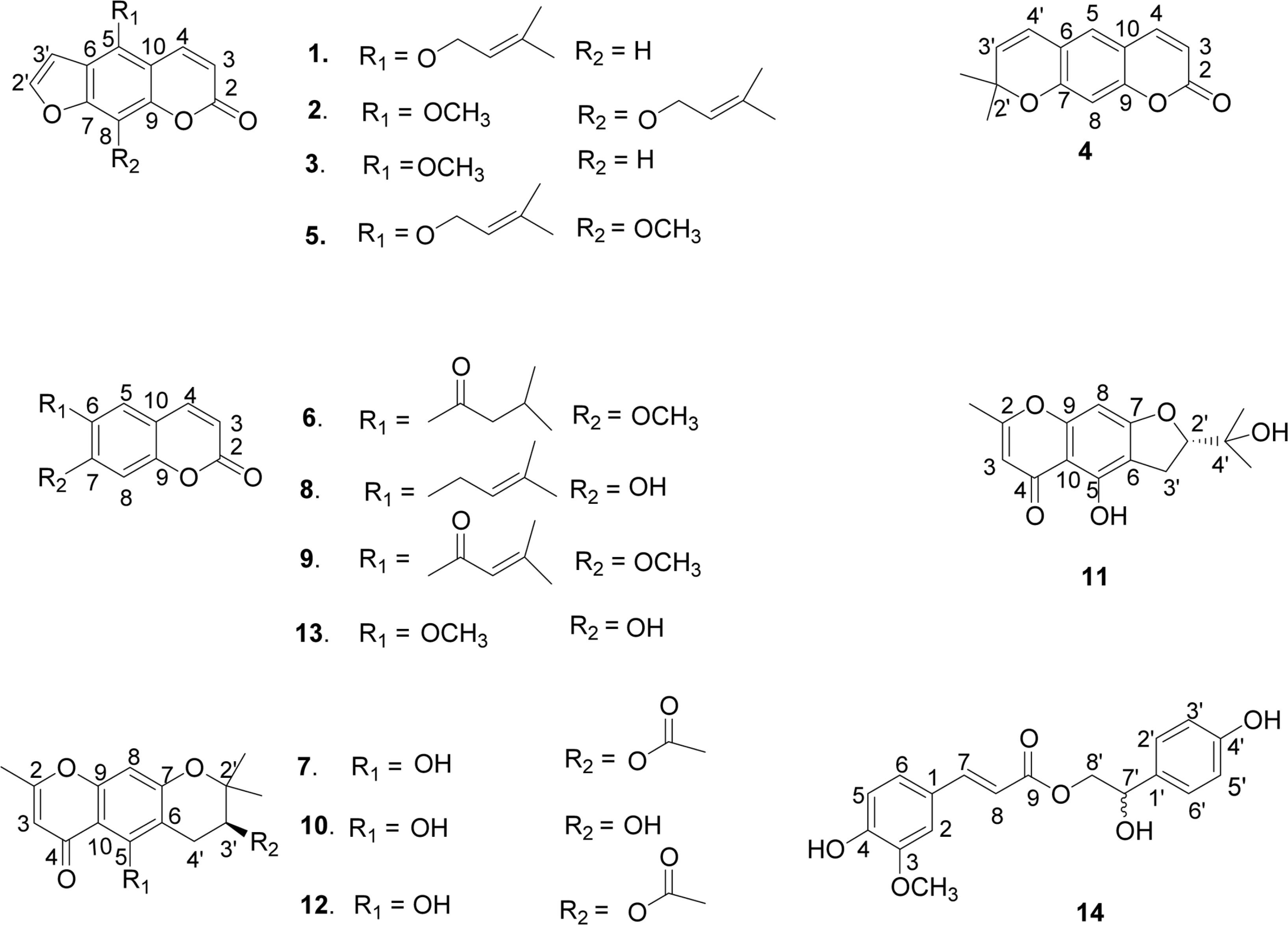
Fourteen compounds were isolated from the stem of Angelica polymorpha. On the basis of spectral data, these compounds were identified as isoimperatorin (1), phellopterin (2), bergapten (3), xanthyletin (4), cnidilin (5), geijerine (6), (−)-3'-acetyl hamaudol (7), 7-demethylsuberosine (8), dehydrogeijerin (9), (–)-hamaudol (10), (+)-visamminol (11), divaricatol (12), scopoletin (13), and decursidate (14), respectively. Among them, compounds 4 – 6, 8, 9, 13, and 14 were isolated for the first time from A. polymorpha. Dehydrogeijerin (6) and geijerin (9) were isolated for the first time from genus Angelica. All isolates tested for inhibitory activity against acetylcholinesterae. Compounds 1 to 13 showed acetylcholinesterase inhibitory activity with IC50 values ranging from 1.4 to 37.5 µM.
Go to : 
References
(1). Lee W. T.Standard illustrations of Korean plants; Academy Pub. Co.: Seoul,. 1996. , p. 254.
(2). Editorial Committee of Zhong Hua Ben Cao of State Administration of Traditional Chinese Medicine of People's Republic of China. Zhong Hua Ben Cao; Shanghai Science and Technology Press: Shanghai,. 1999. 892–893.
(3). Yang Y.., Zhang Y.., Ren F. X.., Yu N. J.., Xu R.., Zhao Y. M.Yao Xue Xue Bao,. 2013. 48:718–722.
(4). Yang S. J.., Yao Q. Q.., Li Y. N.., Chen X. X.., Cui S. X.., Bai S. Y.., Wang F. L. J.Asian Nat. Prod. Res. 2012. 14:76–79.
(5). Dan F. J.., Chu L. J.., Tian Y.., Dong J. X.., Cai Z. J.Shizhen Guoyi Guoyao. 2011. 22:299–300.
(6). Yang Y.., Yu N.., Zhang Y.., Ren F.., Zhang A.., Zhao Y.Zhongguo Yaoxue Zazhi. 2010. 45:1320–1323.
(7). Yang Y.., Yu N.., Liang F.., Zhang Y.., Ren F. X.., Zhang A.., Zhao Y.Pharm. J. Chin. PLA. 2010. 26:189–191.
(8). Han S.., Bai S. Y.., Yang S. J. J.Jining Med. Univ. 2010. 33:13–14.
(9). Li Y.., Yang S.., Bai S.Zhongguo Zhong Yao Za Zhi. 2009. 34:854–857.
(10). Pachaly P.., Kroll-Horstmann A.., Sin K. S.Pharmazie. 2000. 55:777–778.
(11). Coyle J. T.., Price D. L.., DeLong M. R.Science. 1983. 219:1184–1190.
(12). Mukherjoo P. K.., Kumar V.., Mal M.., Houghton P. J.Phytomedicine. 2007. 14:289–300.
(13). Houghton P. J.., Ren Y.., Howes M. J.Nat. Prod. Rep. 2006. 23:181–199.
(14). Anand P.., Singh B.Arch. Pharm. Res. 2013. 36:375–399.
(15). Ellman G. L.., Courtney K. D.., Andres Jr. V.., Feather-Stone R. M.Biochem. Pharmacol. 1961. 7:88–95.
(16). Kim S. J.., Chin Y. W.., Yoon K. D.., Ryu M. Y.., Yang M. H.., Lee J. H.., Kim J. W.Kor. J. Pharmacogn. 2008. 39:357–364.
(17). Wu T. S.., Kuoh C. S.., Furukawa H.Chem. Pharm. Bull. 1983. 31:895–900.
(18). Abreu V. G. C.., Silva M. C.., Magalhâes R. M.., Pilo-Veloso D.., Xavier I. F. S.., Oliveira P. M.., Alcântra A. F. C.Acta Amazonica. 2010. 40:711–718.
(19). Lee K. H.., Soine T. O. J.Pharm. Sci. 1969. 58:675–681.
(20). Frank K.., Porzel A.., Masaoud M.., Adam G.., Schmidt J.Phytochemistry. 2001. 56:611–621.
(21). Fujioka T.., Furumi K.., Fujii H.., Okabe H.., Mihashi K.., Nakano Y.., Matsunaga H.., Katano M.., Mori M.Chem. Pharm. Bull. 1999. 47:96–100.
(22). An R. B.., Park B. Y.., Kim J. H.., Kwon O. K.., Lee J. K.., Min B. S.., Ahn K. S.., Oh S. R.., Lee H. K.Nat. Prod. Sci. 2005. 11:79–84.
(23). Park H. Y.., Kwon S. B.., Heo N. K.., Chun W.., Kim M. J.., Kwon Y. J.Korean Soc. Appl. Biol. Chem. 2011. 54:194–199.
(24). Skalicak-Wo niak K.., Mroczek T.., Garrard I.., G owniak K. J.Sep. Sci. 2012. 35:790–797.
(25). Wang L.., Yu M. M.., Chi Y. Q.., Ouyang W. B.., Zang Z.., Zhao Y.Zhongguo Zhong Yao Za Zhi,. 2014. 39:3969–3973.
(26). Kong L. Y.., Yao N. H.Chin. Chem. Lett. 2000. 11:315–318.
(27). Lee H. K.., Oh S. R.., Kwon O. K.., Ahn K. S.., Lee J.., Kim J. C.., Min B. S.., Joung H.Phytother. Res. 2007. 21:406–409.
(28). Steck W.., Mazurek M.Lloydia. 1972. 35:418–439.
(29). Cairns N.., Harwood L. M.., Astles D. P.Tetrahedron. 1992. 36:7581–7590.
(30). Domínguez X. A.., Cano G.., Luna I.., Dieck A.Phytochemistry,. 1977. 16:1096.
(31). Ritchie E.., Taylor W. C.., Young L. M.Aust. J. Chem. 1968. 21:1381–1382.
(32). Lahey F. N.., Wluka D. J.Aust. J. Chem. 1955. 8:125–128.
(33). Kim, D. K; Lim J. P.., Yang J. H.., Eom D. O.., Eun J. S.., Leem K. H.Arch. Pharm. Res. 2002. 25:856–859.
(34). Lee J. H.., Lee K. T.., Yang J. H.., Baek N. I.., Kim D. K.Arch. Pharm. Res. 2004. 27:53–56.
(35). Kang S. Y.., Lee K. Y.., Sung S. H.., Park M. J.., Kim Y. C. J.Nat. Prod. 2001. 64:683–685.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download