Abstract
A sesquiterpene was purified from Artemisia iwayomogi methanolic extract during the course of searching anti-inflammatory principle from medicinal plants. A sesquiterpene identified as armefolin inhibited the production of nitric oxide (NO) and attenuated inducible nitric oxide synthase (iNOS) protein level in lipopolysaccharide (LPS)-activated RAW 264.7 cells. Armefolin also down-regulated mRNA expressions of iNOS and pro-inflammatory cytokines, interleukin-1β and interleukin-6 in LPS-activated macrophages. Moreover, armefolin suppressed the degradation of inhibitory-κBα (I-κBα) in LPS-activated macrophages. These data suggest that armefolin from A. iwayomogi can suppress the LPS-induced production of NO and the expression of iNOS gene through inhibiting the degradation of I-κBα. Taken together, armefolin from A. iwayomogi might be a candidate as promising anti-inflammatory agent.
Go to : 
References
(1). Kim J. K.Illustrated Natural Drugs Encyclopedia; Namsandang publisher: Seoul,. 1989. , p. 79.
(2). Yan X. T.., Ding Y.., Lee S. H.., Li W.., Sun Y. N.., Yang S. Y.., Jang H. D.., Kim Y. H.Nat. Prod. Sci. 2014. 20:176–181.
(3). Ha H.., Lee H.., Seo C. S.., Lim H. S.., Lee M. Y.., Lee J. K.., Shin H.Evid. Based Complement. Alternat. Med. 2014. 2014:673286.
(4). Wang J. H.., Choi M. K.., Shin J. W.., Hwang S. Y.., Son C. G. J.Ethnopharmacol. 2012. 140:179–185.
(5). Yu H. H.., Kim Y. H.., Kil B. S.., Kim K. J.., Jeong S. I.., You Y. O.Planta Med. 2003. 69:1159–1162.
(6). Kim A. R.., Zou Y. N.., Park T. H.., Shim K. H.., Kim M. S.., Kim N. D.., Kim J. D.., Bae S. J.., Choi J. S.., Chung H. Y.Phytother. Res. 2004. 18:1–7.
(7). Ahn H.., Kim J. Y.., Lee H. J.., Kim Y. K.., Ryu J. H.Arch. Pharm. Res. 2003. 26:301–305.
(8). Nathan C.., Xie Q. W. J.Biol. Chem. 1994. 269:13725–13728.
(10). Weinberg J. B.., Fermor B.., Guilak F.Subcell. Biochem. 2007. 42:31–62.
(11). Mittal R.., Gonzalez-Gomez I.., Goth K. A.., Prasadarao N. V.Am. J. Pathol. 2010. 176:1292–1305.
(12). Kapur S.., Picard F.., Perreault M.., Deshaies Y.., Marette A.Int. J. Obes. Relat. Metab. Disord. 2000. 24:S36–S40.
(13). Förstermann U.., Schmidt H. H.., Pollock J. S.., Sheng H.., Mitchell J. A.., Warner T. D.., Nakane M.., Murad F.Biochem. Pharmacol. 1991. 42:1849–1857.
(14). Förstermann U.., Pollock J. S.., Tracey W. R.., Nakane M.Methods Enzymol. 1994. 233:258–264.
(15). Hiraku Y.., Kawanishi S.., Ichinose T.., Murata M. Ann. N. Y.Acad. Sci. 2010. 1203:15–22.
(16). Hiraku Y.., Kawanishi S.Methods Mol. Biol. 2009. 512:3–13.
(17). Mata R.., Delgado G.., de Vivar A. R.Phytochemistry. 1984. 23:1665–1668.
(18). Gasparini C.., Feldmann M.Curr. Pharm. Des. 2012. 18:5735–5745.
(19). Kanarek N.., Ben-Neriah Y.Immunol. Rev. 2012. 246:77–94.
(20). Valera F. C.., Umezawa K.., Brassesco M. S.., Castro-Gamero A. M.., Queiroz R. G. D. P.., Scrideli C. A.., Tone L. G.., Anselmo-Lima W. T.Cell. Physiol. Biochem. 2012. 30:13–22.
(21). Ganai A. A.., Khan A. A.., Malik Z. A.., Farooqi H.Toxicol. Appl. Pharmacol. 2015. 283:139–146.
(22). Kaminska B.Biochim. Biophys. Acta. 2005. 1754:253–262.
(23). Kim E. K.., Choi E. J.Arch. Toxicol. 2015. 89:867–882.
Go to : 
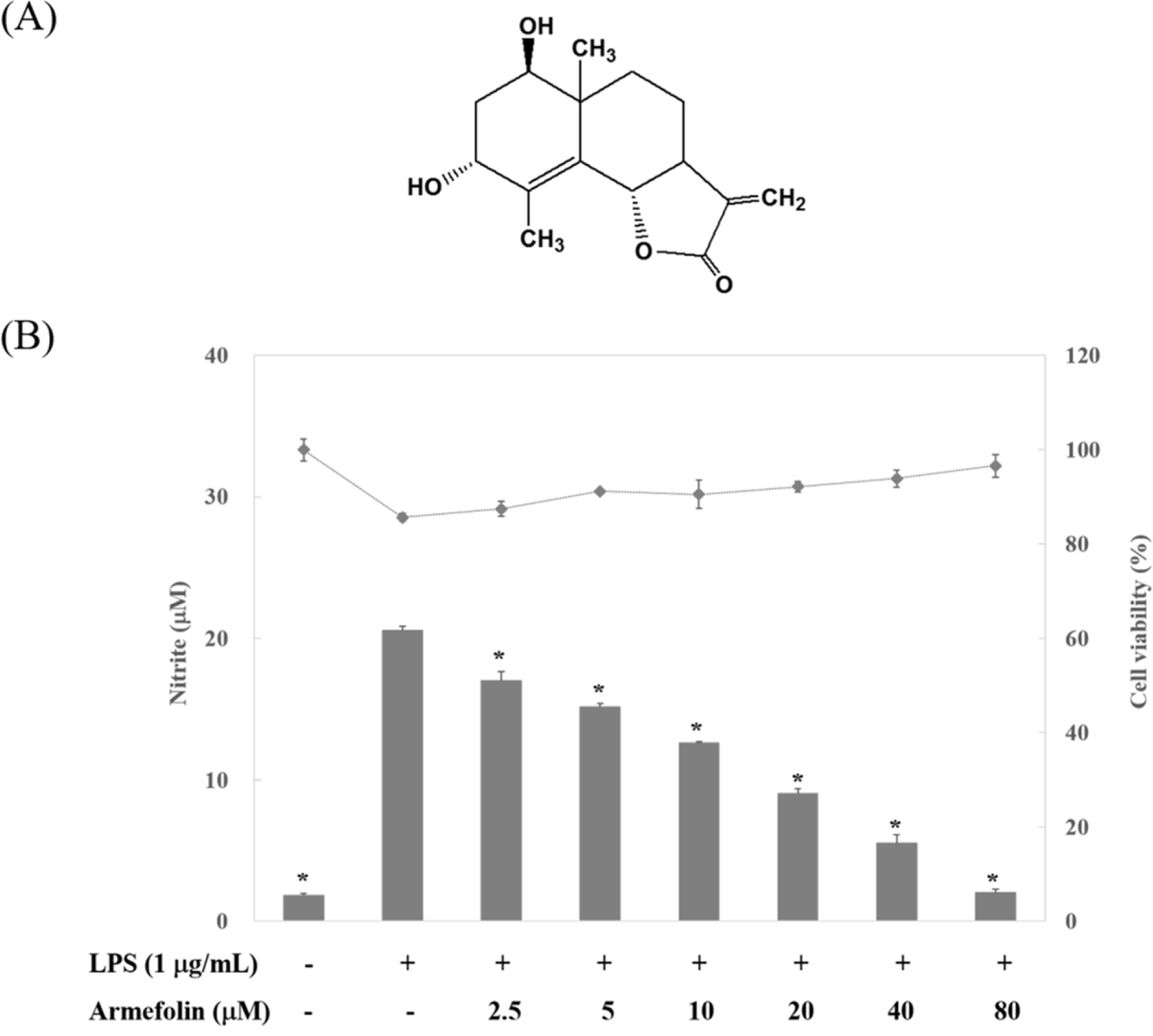 | Fig. 1.The structure of armefolin purified from A. iwayomogi (A) and the effect of armefolin on LPS-induced NO production in RAW 264.7 macrophages (B). The amount of nitrite in culture medium was measured by using Griess reagents (bar). Cell viability was determined by MTT assay (•). The values are expressed as the means ± S.D. of three individual experiments. ∗ p < 0.05 indicate significant differences from the LPS alone. |
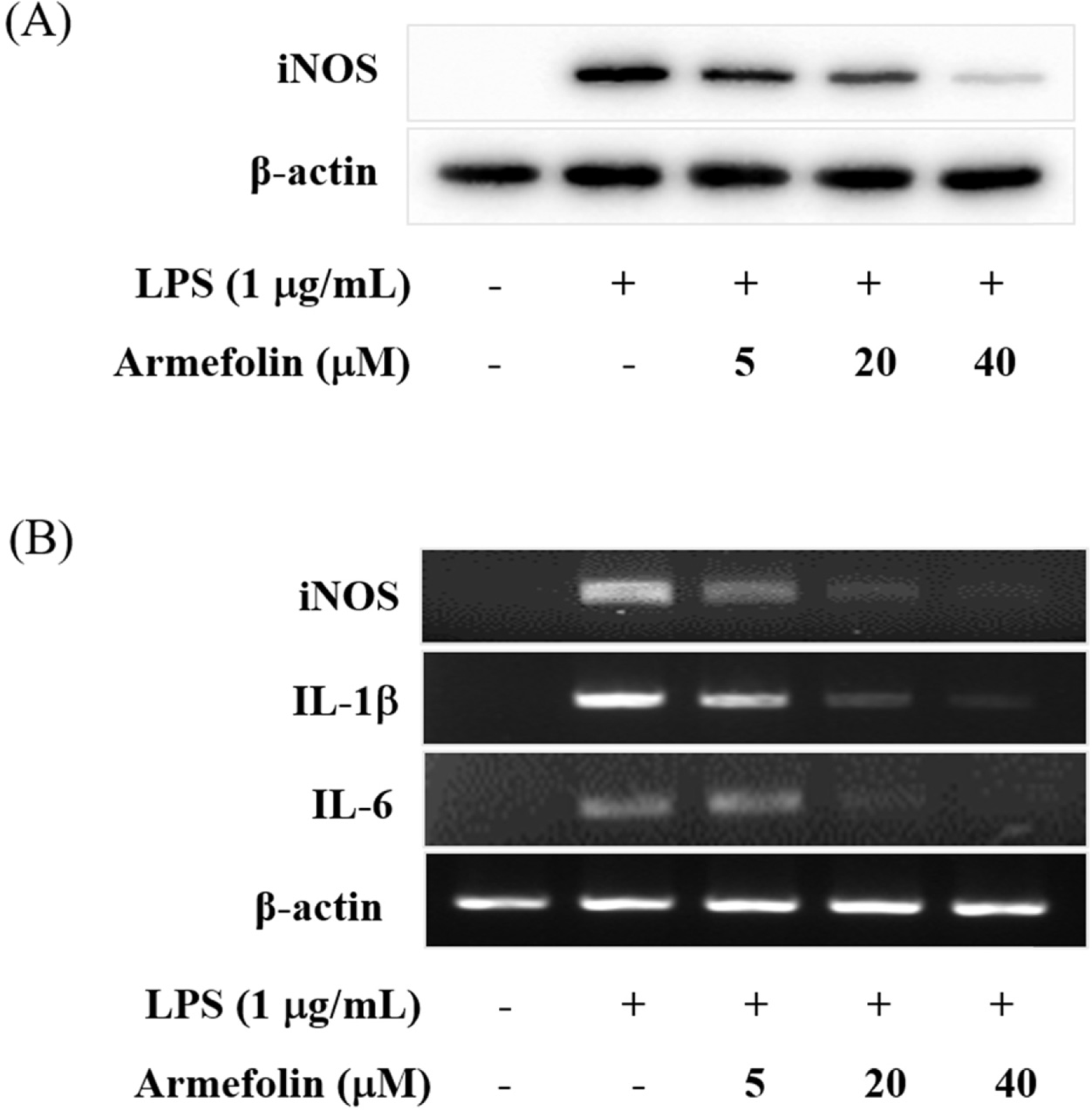 | Fig. 2.Effects of armefolin on iNOS expression (A) and inflammatory cytokines (B) in LPS-activated RAW 264.7 macrophages. Cells were treated with armefolin for 20 h during LPS (1 μg/mL) activation. Cell lysates were prepared and the iNOS and β-actin protein levels were determined by Western blotting (A). Cells were treated with armefolin for 6 h during LPS (1 μg/ mL) activation. The mRNA levels for iNOS, IL-1β, IL-6 and β-actin were determined by RT-PCR from total RNA extracts (B). β-Actin was used as an internal control. Images are the representative of three independent experiments that shows similar results. |
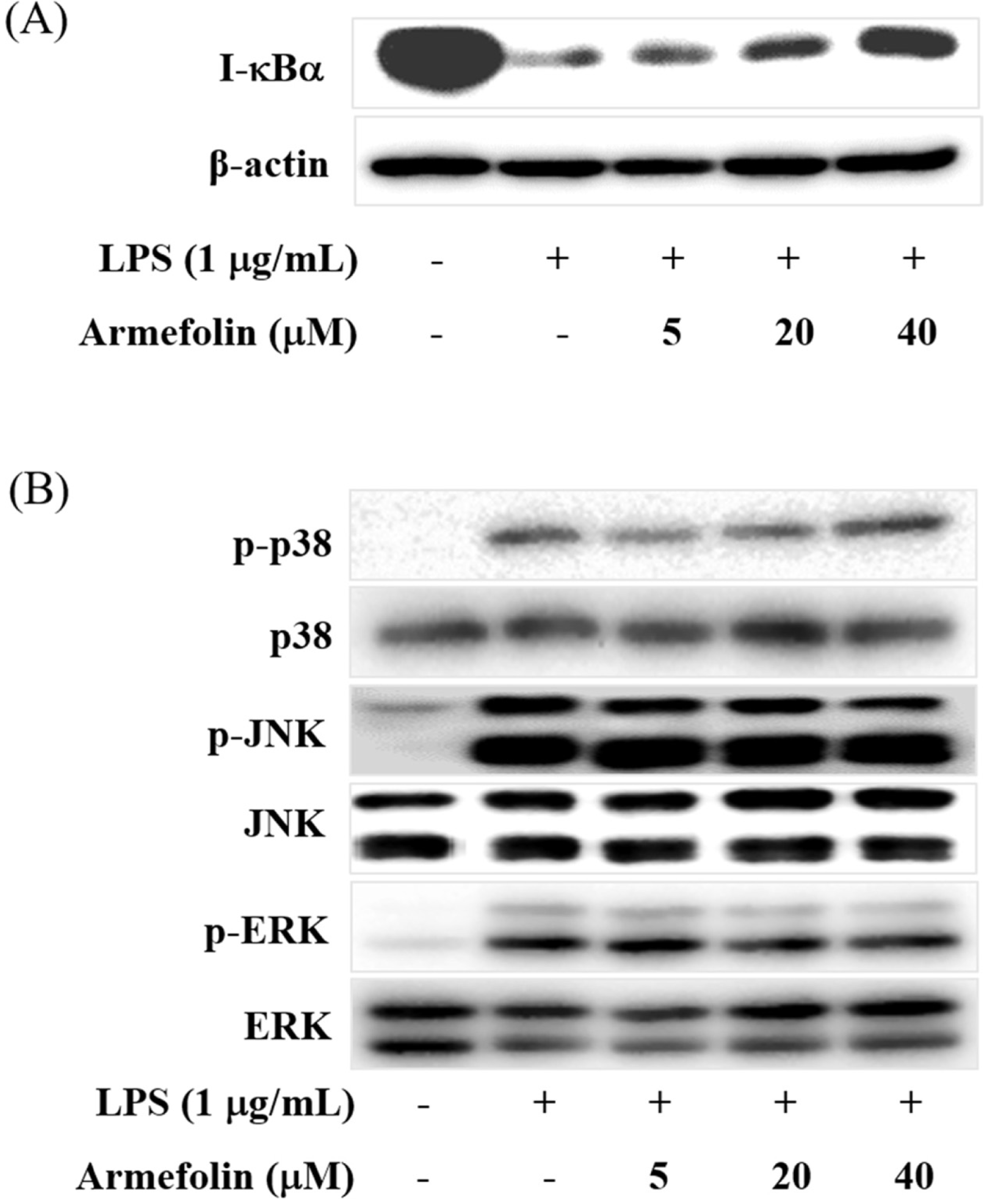 | Fig. 3.The effects of armefolin on LPS-induced I-κBα degradation (A) and phosphorylation of p38, JNK and ERK (B) in RAW 264.7 macrophages. Cells were pre-treated with armefolin for 30 min prior to LPS treatment. Cell lysates were prepared after LPS treatment for an additional 15 min, and then levels of degraded I-κBα (A), phosphorylated p38, JNK and ERK (B) were determined by Western blot. Images are the representative of three independent experiments that shows similar results. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download