Abstract
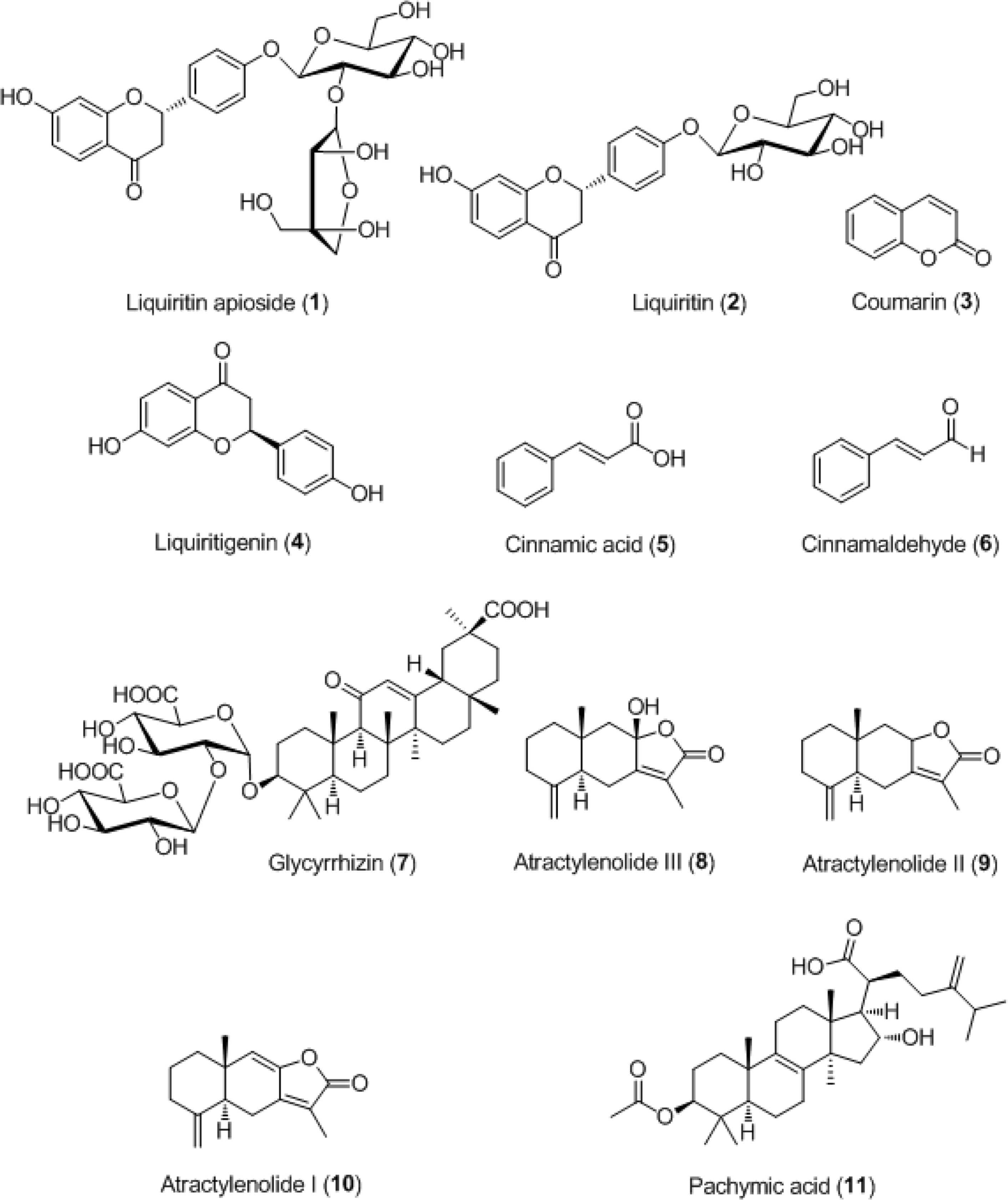
This study proposes a sensitive and selective liquid chromatography-electrospray ionization tandem mass spectrometry method of efficiently assessing the quality of a traditional herbal medicine called Yeonggyechulgam-tang (YGCGT). The following compounds 1 – 11, namely, liquiritin apioside (1), liquiritin (2), liquiritigene (3), coumarin (4), cinnamic acid (5), cinnamaldehyde (6), glycyrrhizin (7), atractylenolide III (8), atractylenolide II (9), atractylenolide I (10), and pachymic acid (11) were separated on a UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm) at a column temperature of 45oC eluted with a gradient condition of 0.1% (v/v) formic acid in distilled water and acetonitrile. The correlation coefficient of the calibration curve of the eleven constituents was ≥ 0.9936. The limits of detection and quantification of the compounds 1 – 11 were 0.06 – 4.73 ng/mL and 0.17–14.20 ng/ mL, respectively. Using this analytical method, the compound 11 in lyophilized YGCGT decoction extract was not detected, while the compounds 1 – 10 were detected 0.13–166.43 mg/g.
Go to : 
References
(1). Zhang J.., Qian C. C.., Hao W.S.; Shang Han Lun; People's Medical Publishing House: Beijing,. 2005. ;p.41–42.
(2). Heo, J. ; Dongeuibogam; Namsandang: Seoul,. 2004. ;p. 128.
(3). Xi F.., Sang F.., Zhou C.., Ling Y.Neural Regener. Res. 2012. 7:2867–2873.
(4). Yuanyuan W.., Minghua J.., Lina Z.., Suhua L.., Jiayu Z.., Yongzhi S.., Chunyu C.., Jian Q. J.Tradit. Chin. Med. 2015. 35:218–221.
(5). Fu S.., Zhang J.., Gao X.., Xia Y.., Ferrelli R.., Fauci A.., Guerra R.., Hu L.Heart Asia. 2010. 2:24–27.
(6). Huang J.., Wang L.., Shi H.., Hou X. J.Tradit. Chin. Med. 2013. 33:343–348.
(7). Kim T. H.., Yang K. S.., Park S. A.Kor. J. Pharmacogn. 1999. 30:12–17.
(8). Park, S. A; Kim T. H.., Yang K. S.Kor. J. Pharmacogn. 2000. 31:364–372.
(9). Kim S.H.; The guideline on the visual and organoleptic examination of herbal medicine II; National Institute of Food and Drug Safety Evaluation: Seoul,. 2007. ;p. 19.
(10). Moon C.J.; The guideline on the visual and organoleptic examination of herbal medicine I; Ministry of Food and Drug Safety: Seoul,. 2006. ;p. 17. , 52, 54.
(11). Li L.., Liang S.., Du, F; Li C. J.Am. Soc. Mass Spectrom. 2007. 18:778–782.
(12). Tan G.., Zhu Z.., Jing J.., Lv L.., Lou Z.., Zhang G.., Chai Y.Biomed. Chromagogr. 2011. 25:913–924.
(13). Concannon S.., Ramachandran V. N.., Smyth W. F.Rapid Commun. Mass Spectrom. 2000. 14:1157–1166.
(14). Wu L. F.., Wang K. F.., Mao X.., Liang W. Y.., Chen W. J.., Li S.., Qi Q.., Cui Y. P.., Zhang L. Z.Molecules. 2016. 21:, 227,. doi: 10.3390/molecules21020227.
(15). Gardana C.., Scaglianti M.., Pietta P.., Simonetti P. J.Pharm. Biomed. Anal. 2007. 45:390–399.
(16). Flamini R.., Vedova, A. D,; Cancian D.., Panighel A.., De Rosso M. J.Mass Spectrom. 2007. 42:641–646.
(17). Chen L.., Qi J.., Chang Y.., Zhu D.., Yu B. J.Pharm. Biomed. Anal. 2009. 50:127–137.
Go to : 
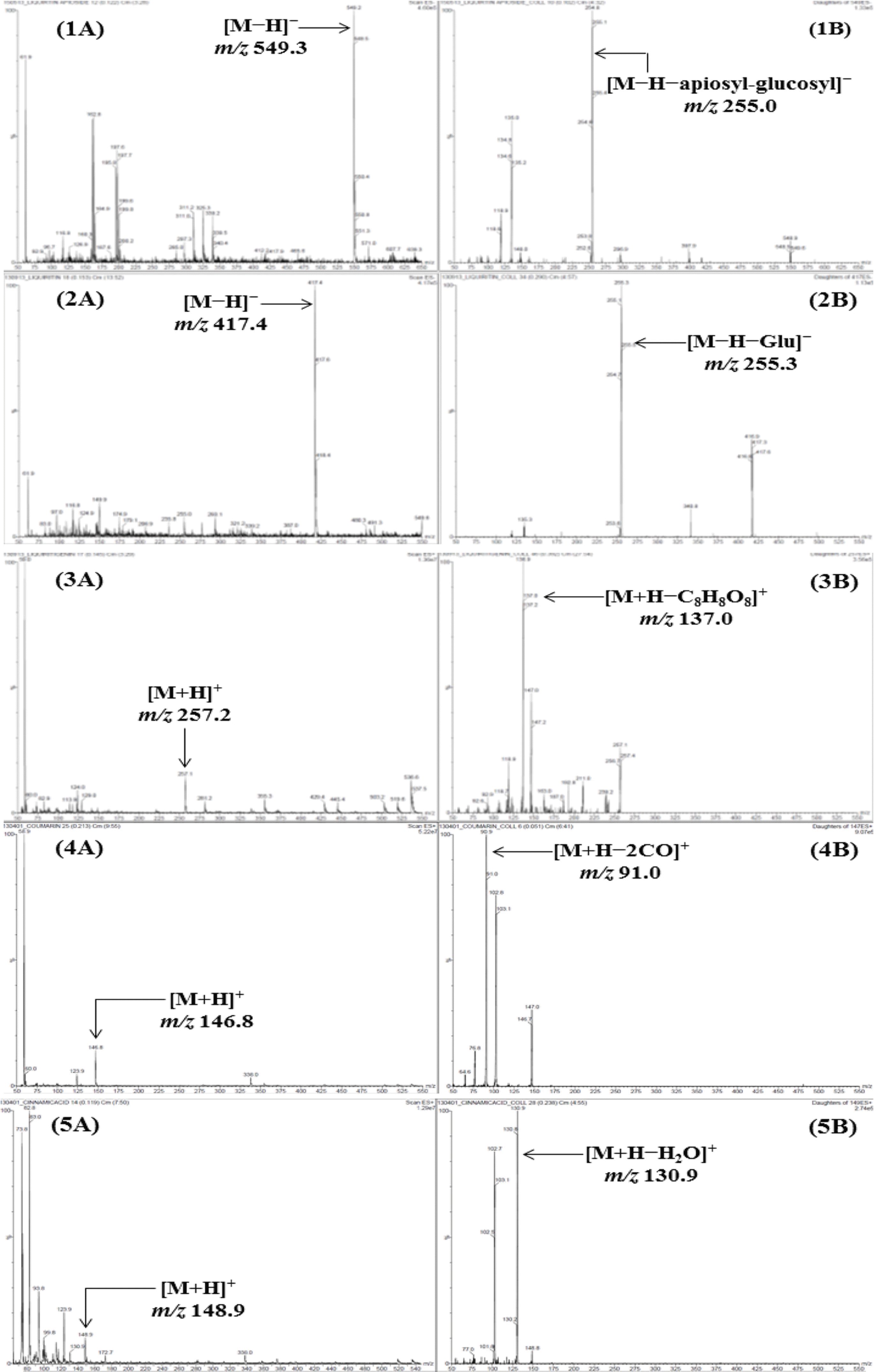 | Fig. 2.Mass spectra of the precursor ion (Q1, A) and product ion (Q3, B) for LC-MS/MS MRM mode of the compounds 1 – 11. Liquiritin apioside (1), liquiritin (2), liquiritigene (3), coumarin (4), cinnamic acid (5), cinnamaldehyde (6), glycyrrhizin (7), atractylenolide III (8), atractylenolide II (9), atractylenolide I (10), and pachymic acid (11). |
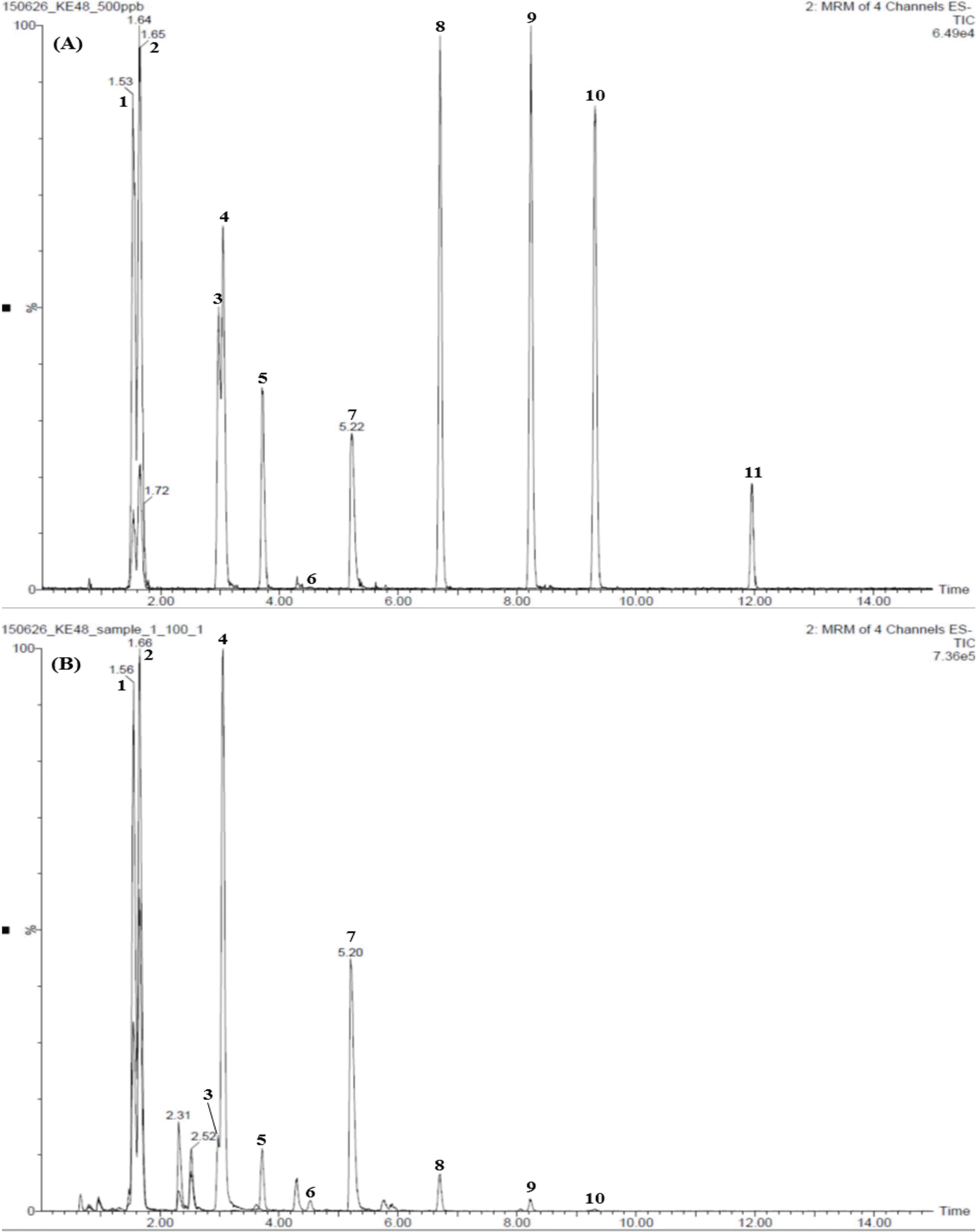 | Fig. 3.Total ion chromatograms of the reference standard (A) and lyophilized Yeonggyechulgam-tang extract (B) by LC-MS/MS MRM mode. Liquiritin apioside (1), liquiritin (2), liquiritigene (3), coumarin (4), cinnamic acid (5), cinnamaldehyde (6), glycyrrhizin (7), atractylenolide III (8), atractylenolide II (9), atractylenolide I (10), and pachymic acid (11, not detected). |
Table 1.
Composition of Yeonggyechulgam-tang
Table 2.
Linearities, regression equation, correlation coefficients, LOD, and LOQ for the compounds 1 – 11
| Analyte | Linear range (ng/mL) | Regression equationa | Correlation coefficient | LODb (ng/mL) | LOQc (ng/mL) |
|---|---|---|---|---|---|
| 1 | 10–500 | y = 8.21x – 27.85 | 0.9997 | 0.55 | 1.65 |
| 2 | 10–500 | y = 9.01x – 19.10 | 1.0000 | 1.14 | 3.41 |
| 3 | 10–500 | y = 29.58x – 29.21 | 1.0000 | 0.11 | 0.34 |
| 4 | 10–500 | y = 38.59x + 51.41 | 0.9999 | 0.91 | 2.74 |
| 5 | 10–500 | y = 21.35x – 67.24 | 0.9999 | 0.87 | 2.60 |
| 6 | 10–500 | y = 1.15x – 18.20 | 0.9936 | 4.73 | 14.20 |
| 7 | 10–500 | y = 3.08x – 60.81 | 0.9961 | 1.42 | 4.27 |
| 8 | 10–500 | y = 52.12x – 97.00 | 1.0000 | 0.15 | 0.44 |
| 9 | 10–500 | y = 56.54x + 33.78 | 1.0000 | 0.06 | 0.17 |
| 10 | 10–500 | y = 53.42x + 63.53 | 1.0000 | 0.12 | 0.37 |
| 11 | 10–500 | y = 1.46x – 10.67 | 1.0000 | 1.05 | 3.16 |
Table 3.
Mass detection condition of the compounds 1 – 11
Table 4.
Amounts of the compounds 1 – 11 in lyophilized Yeonggyechulgam-tang extract (n = 3)
| Compound | Amount (mg/g) | Source | ||
|---|---|---|---|---|
| Mean | SD | RSD (%) | ||
| 1 | 81.58 | 1.28 | 1.56 | G. uralensis |
| 2 | 79.88 | 0.31 | 0.39 | G. uralensis |
| 3 | 8.96 | 0.27 | 3.02 | G. uralensis |
| 4 | 54.52 | 1.45 | 2.65 | C. cassia |
| 5 | 10.18 | 0.25 | 2.50 | C. cassia |
| 6 | 121.09 | 1.11 | 0.92 | C. cassia |
| 7 | 166.43 | 11.17 | 6.71 | G. uralensis |
| 8 | 2.47 | 0.04 | 1.58 | A. macrocephala |
| 9 | 0.88 | 0.03 | 2.94 | A. macrocephala |
| 10 | 0.13 | 0.01 | 4.44 | A. macrocephala |
| 11 | N.D.a | – | – | P. cocos |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download