Abstract
A new phenol and hydrazide derivatives were obtained for the first time from the C. giganteawhite by silica gel column chromatography. The structure of the isolated compounds was identified by UV, IR NMR and MS. C. gigantea was scientifically reported for several medicinal properties viz. analgesic, antimicrobial and cytotoxic. In this screening work, anti-microbial activity of test compounds was found to be active against all organisms. Additionally, anti-inflammatory activity of the test groups has reduced the thickness of edema of the hind paw compared to the control group.
Go to : 
References
(1). Subramanian S. P.., Saratha V. J.Pharm. Res. 2010. 3:517–521.
(2). Kirtikar K. R.., Basu B.D.Indian Medicinal Plants: Volume III. 2nd ed. International Book Distributors: India;1999. p. 191–192.
(3). Habib M. R.., Karim M. R.Microbiology. 2009. 37:31–36.
(4). Chitme H.R.; Chandra, M.; Kaushik, S.J. Pharm. Pharm. Sci. 2004. 7:70–75.
(6). Jeyachandran R.., Mahesh A.Res. J. Micro. 2007. 2:645–649.
(7). Shah P. M.Clin. Microbiol. Infect. 2005. 11:36–42.
(8). Nair R.., Chanda S.Indian J. Pharmacol. 2006. 38:142–144.
(9). Denko C.W.Biochemistry of Inflammation: The role of leukocyte chemotaxis in inflammation;. Springer Netherlands: London;1992. p. 177–181.
(10). Hajhashemi V.., Sajjadi S. E.., Heshmati M.V.J. Ethnopharmacol. 2009. 124:475–480.
(11). Bauer A.W.., Kirby W.M.., Sherries J. C.., Turck M.Am. J.Clin.Pathol. 1966. 45:493–496.
(12). Wright D.A.., Payne J.P.Br. J.Anesth. 1962. 34:379–385.
(13). Leite L.F.., Ramos M. N.., da Silva J.B.., Miranda A.L.., Fraga C.A.., Barreiro E. J.Farmaco. 1999. 54:747–757.
(14). Lima P.C.., Lima L.M.., da Silva K. C.., Léda P. H.., de Miranda A. L.., Fraga C.A.., Barreiro E. J.Eur. J. Med. Chem. 2000. 35:187–203.
(15). Loncle C.., Brunel J.M.., Vidal N.., Dherbomez M.., Letourneux Y.Eur. J. Med. Chem. 2004. 39:1067–1071.
(16). Todeschini A.R.., De Miranda A.L.P.., Da Silva K. C. M.., Parrini S. C.., Barreiro E. J.Eur. J. Med. Chem. 1998. 33:189–199.
(17). Terzioglu N.., Gürsoy A.Eur. J. Med. Chem. 2003. 38:781–786.
(18). Abadi H.., Eissa A.A.., Hassan G. S.Chem. Pharm. Bull. 2003. 51:838–844.
(19). Kumar D.., Maruthi Kumar N.., Ghosh S.., Shah K.Bioorg. Med. Chem. Lett. 2012. 22:212–215.
(20). Ofner J.., Krüger H. U.., Grothe H.., Schmitt-Kopplin P.., Whitmore K.., Zetzsch C.Atmos. Chem. Phys. 2011. 11:1–15.
(21). Ban H. Y.., Li C. M.ActaCryst. Sec. 2008. E64:2260.
(22). Liu L.., Alam M. S.., Lee D. U.Bull. Korean Chem. Soc. 2012. 33:3361–3367.
(23). Yin H.D.., Hong M.., Wang Q.B.., Xue S.C.., Wang D. Q. J.Organo.Chem. 2005. 690:1669–1676.
(24). Yin H.D.., Chen S. W.Inorg. Chim. Acta. 2006. 359:3330–3338.
(25). Gangoué-Piéboji J.., Pegnyemb D.E.., Niyitegeka D.., Nsangou A.., Eze N.., Minyem C.., Mbing J. N.., Ngassam P.., Tih R. G.., Sodengam B. L.., Bodo B.Ann. Trop. Med. Parasitol. 2006. 100:237–243.
(26). Shan B.., Cai Y.Z.., Brooks J. D.., Corke H.Int. J. Food Microbiol. 2007. 117:112–119.
(27). Katzung B. G.Basic and Clinical pharmacology: 9thedn; McGraw Hill: London,. 2004. 641–646.
(28). Winter C. A.., Risley E. A.., Nuss G. W.Proc. Soc. Exp. Biol. Med. 1962. 111:544–547.
(29). Turner R. A.Screening methods in pharmacology;. Academic Press: New York;1965. , p. p. 158.
(30). Vinegar R.., Truax J. F.., Selph J. L.., Johnston P. R.., Venable A.L.; Mckenzie, K.K. Fed. Proc. 1987. 46:118–126.
Go to : 
Table 1.
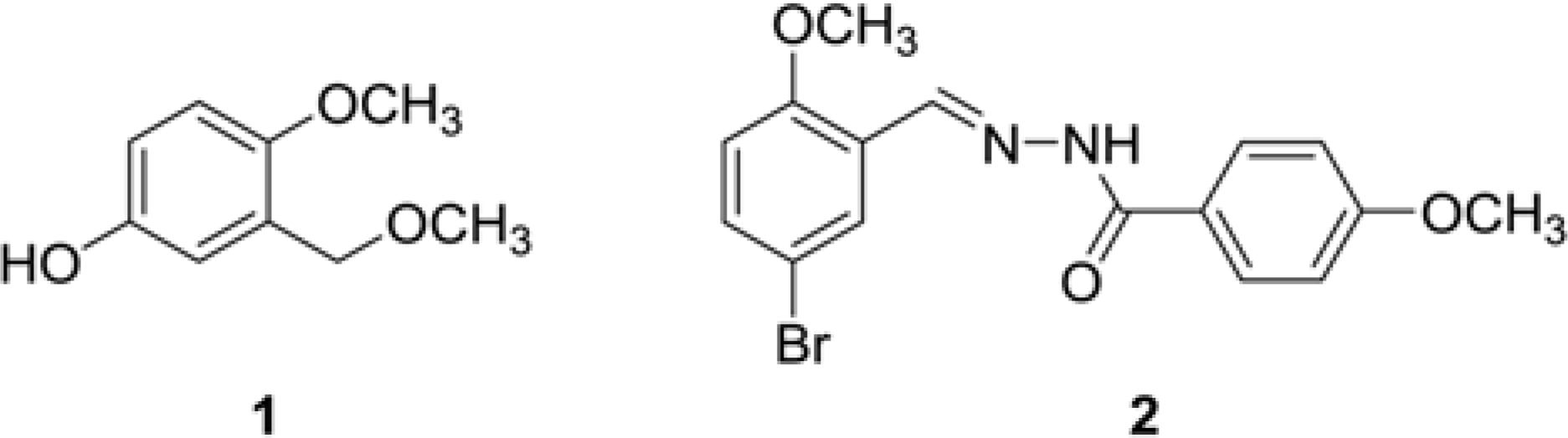
Anti-microbial activity of phenol 4-methoxy-3-(methoxymethyl) phenol (1) and (E)-N'-(5-bromo-2-methoxybenzylidene)-4-methoxy benzohydrazide (2) extracted from Calotropis gigantea white
| Micro organism | Zone of inhibition mm in diameter | ||||
|---|---|---|---|---|---|
| Compound 1 | Compound 2 | Standard Antibiotic | |||
| Concentration in µg/ml | |||||
| 100 | 200 | 100 | 200 | ||
| Escherichia coli (MTCC 62) | 08 ± 0.98 | 17 ± 1.28 | 07 ± 0.84 | 14 ± 1.34 | 18 ± 1.22∗ |
| Staphylococcus aureus (MTCC 87) | 05 ± 0.65 | 13 ± 1.85 | 04 ± 0.24 | 11 ± 1.74 | 17 ± 1.98∗ |
| Aspergillus flavus (MTCC 96) | 09 ± 0.36 | 14 ± 1.06 | 02 ± 0.88 | 09 ± 1.08 | 12 ± 1.04∗∗ |
| Aspergillus niger (MTCC 107) | 10 ± 0.65 | 13 ± 1.55 | 05 ± 0.75 | 08 ± 1.57 | 12 ± 1.50∗∗ |
| Candida albicans (MTCC 118) | 09 ± 0.30 | 16 ± 1.50 | 04 ± 0.45 | 12 ± 1.50 | 22 ± 1.60∗∗ |
Table 2.
Anti-inflammatory activity of phenol 4-methoxy-3-(methoxymethyl) phenol (1) and (E)-N'-(5-bromo-2-methoxybenzylidene)-4-methoxy benzohydrazide (2) extracted from Calotropis gigantea white




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download