Abstract
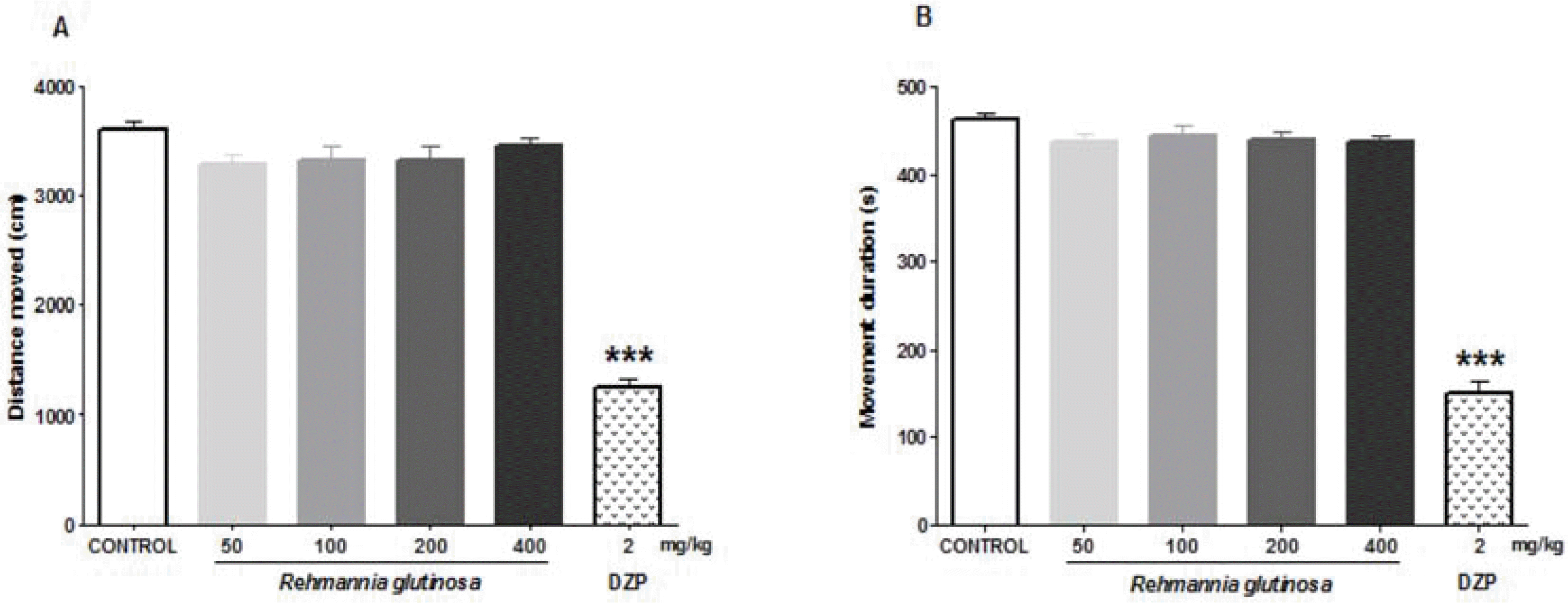
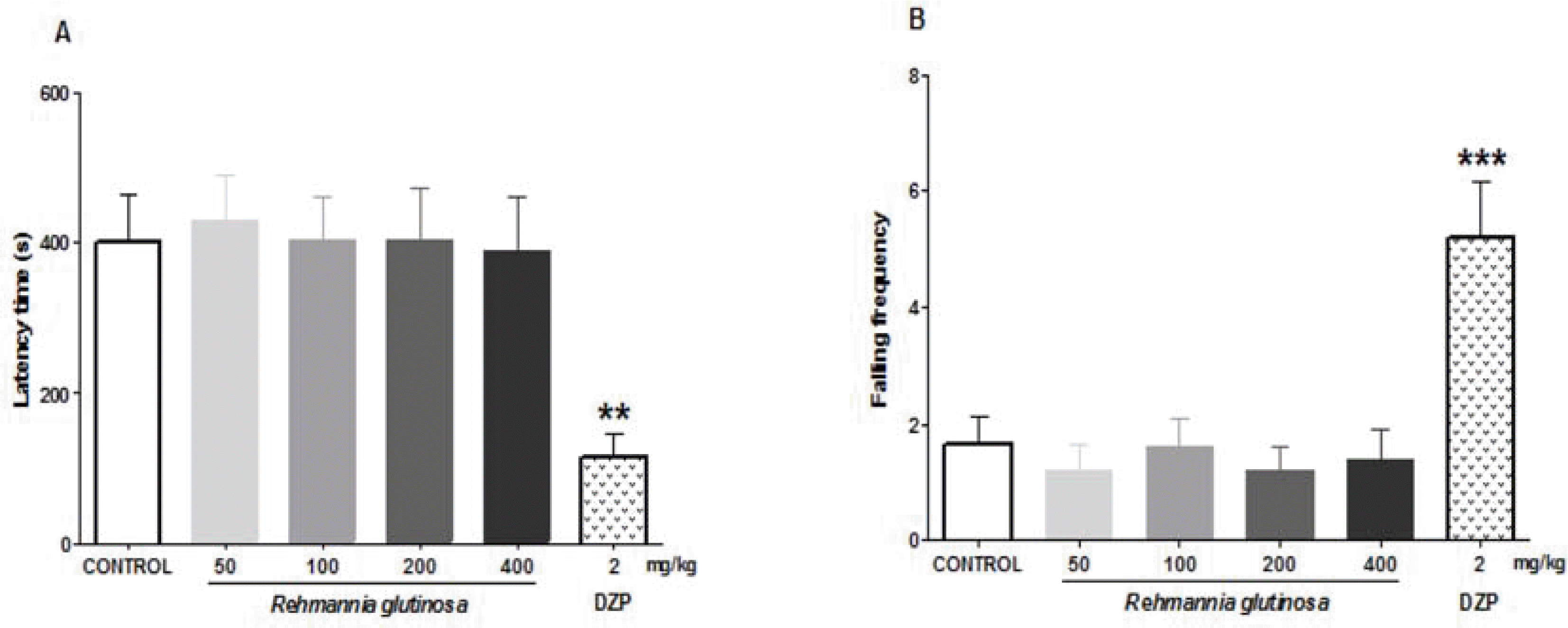
Epilepsy is a brain disorder that affects millions of people worldwide. It is characterized by recurrent and unpredictable seizures that are usually controlled with antiepileptic/anticonvulsive drugs. However, most antiepileptic drugs produce various side effects such as tolerance and sedation. Thus, there is a growing interest for alternative anticonvulsive drugs, preferably from natural or herbal sources. In this study, we evaluated the anticonvulsive effects of Rehmannia glutinosa (RG). The anticonvulsive effect of RG extract was evaluated using electroshock- and chemical-induced seizure tests in mice. To identify its probable mechanism of action, the effects of RG extract on Cl− influx was measured in vitro. We found that RG extract has anticonvulsive effects against electroshock-induced seizures, as indicated by an increased seizure threshold in mice. The RG extract also decreased the percentage of seizure responses induced by the GABAergic antagonist, pentylenetetrazole. These results suggest that the anticonvulsive effects of RG extract are mediated through a GABAergic mechanism. In support of this mechanism, our in vitro test showed that RG extract increases intracellular Cl− influx. Furthermore, RG extract did not show sedative and/or muscle relaxant effects in the open-field and rota-rod tests. Altogether, these results confirm that RG extract could be a herbal anticonvulsant and a potential alternative for clinical use.
Go to : 
References
(1). Fisher R.S.., van Emde Boas W.., Blume W.., Elger C.., Genton P.., Lee P.., Engel Jr J.Epilepsia. 2005. 46:470–472.
(2). Blume W.T.., Lüders H.O.., Mizrahi E.., Tassinari C.., van Emde Boas W.., Engel Jr J.Epilepsia. 2001. 42:1212–1218.
(3). Shorvon S.Handbook of epilepsy treatment (3rd ed); John Wiley & Sons: United Kingdom,. 2010.
(4). Schmidt D.., Schachter S.C.BMJ. 2014. 348:g254.
(5). Swann A.C. J.Clin. Psychiatry. 2001. 62:16–21.
(6). Megiddo I.., Colson A.., Chisholm D.., Dua T.., Nandi A.., Laxminarayan R.Epilepsia. 2016. 57:464–474.
(7). Samuels N.., Finkelstein Y.., Singer S.R.., Oberbaum M.Epilepsia. 2008. 49:373–380.
(8). QuintansJúnior L.J.., Almeida J.R.., Lima J.T.., Nunes X.P.., Siqueira J.S.., Oliveira L.E.G.., Almeida R.N.., Athayde-Filho P.F.., Barbosa-Filho J.M.Rev. Bras. Farmacogn. 2008. 18:798–819.
(9). Woo T.S.., Yoon S.Y.., dela Peña I.C.., Choi J.Y.., Lee H.L.., Choi Y.J.., Lee Y.S.., Ryu J.H.., Choi J.S.., Cheong J.H.Biomol. Ther. 2011. 19:342–347.
(10). Hijikata Y.., Yasuhara A.., Yoshida Y.., Sento S. J.Altern. Complement. Med. 2006. 12:673–677.
(11). Kwon B.H.., Gu B.S. J.Oriental Neuropsychiatry,. 1999. 10:1–27.
(12). Jiao Z.., Cheng Y.., Wang H.., Lei C.., Wang G.G.., Han L.Appl. Plant Sci. 2015. 3:1500054.
(13). Baek G.H.., Jang Y.S.., Jeong S.I.., Cha J.., Joo M.., Shin S.W.., Ha K.T.., Jeong H. S.Inflammation. 2012. 35:1232–1241.
(14). Dingding C.., Dai Dezai Z. T.Pharmacology and Clinics of Chinese Materia Medica. 1996. 5:010.
(15). Huang Y.., Jiang C.., Hu Y.., Zhao, X.;Shi C.., Yu Y.., Liu C.., Tao Y.., Pan H.., Feng Y.., Liu J.., Wu Y.., Wang D.Carbohydr. Polym. 2013. 96:516–521.
(16). Kim H.M.; An, C.S.; Jung, K.Y.; Choo, Y.K.; Park, J.K.; Nam, S.Y. Pharmacol. Res. 1999. 40:171–176.
(17). Kim S.S.; Son, Y.O.; Chun, J.C.; Kim, S.E.; Chung, G.H.; Hwang, K.J.; Lee, J.C. Redox. Rep. 2005. 10:311–318.
(18). Wang Y.B.., Liu Y.F.., Lu X.T.., Yan F.F.., Wang B.., Bai W.W.., Zhao Y.X.PLoS One. 2013. 8:, e54303.
(19). Yuan Y.., Hou S.., Lian T.., Han Y.Zhongguo Zhong Yao Za Zhi. 1992. 17:366–368.
(20). Zhang R.X.., Li M.X.., Jia Z.P. J.Ethnopharmacol. 2008. 117:199–214.
(21). Yoon S.Y.., dela Peña I.C.., Shin C.Y.., Son K.H.., Lee Y.S.., Ryu J.H.., Cheong, J.H.;Ko K.H.Eur. J. Pharmacol. 2011. 659:155–160.
(22). Pellmar T. C.., Wilson W.A.Science. 1977. 197:912–914.
(23). Larson A.A.., Beitz A. J. J.Neurosci. 1988. 8:3822–3826.
(24). West M. R.., Molloy C. R.Anal. Biochem. 1996. 241:51–58.
(25). Löscher W.., Fassbender C. P.., Nolting B.Epilepsy Res. 1991. 8:79–94.
(26). Rogawski M.A.., Porter R.J.Pharmacol. Rev. 1990. 42:222–286.
(27). De Deyn P.P.., Marescau B.., MacDonald R.L.Acta Neurol. Belg. 1989. 90:65–81.
(29). Young A.B.., Zukin S.R.., Snyder S.H.Proc. Natl. Acad. Sci. U.S.A. 1974. 71:2246–2250.
(30). Rho J.M.., Donevan S.D.., Rogawski M.A.Ann. Neurol. 1994. 35:229–234.
(31). Crestani F.., Löw K.., Keist R.., Mandelli M.J.., Möhler H.., Rudolph U.Mol. Pharmacol. 2001. 59:442–445.
(32). McBurney R.N.., Barker J. L.Nature. 1978. 274:596–597.
Go to : 
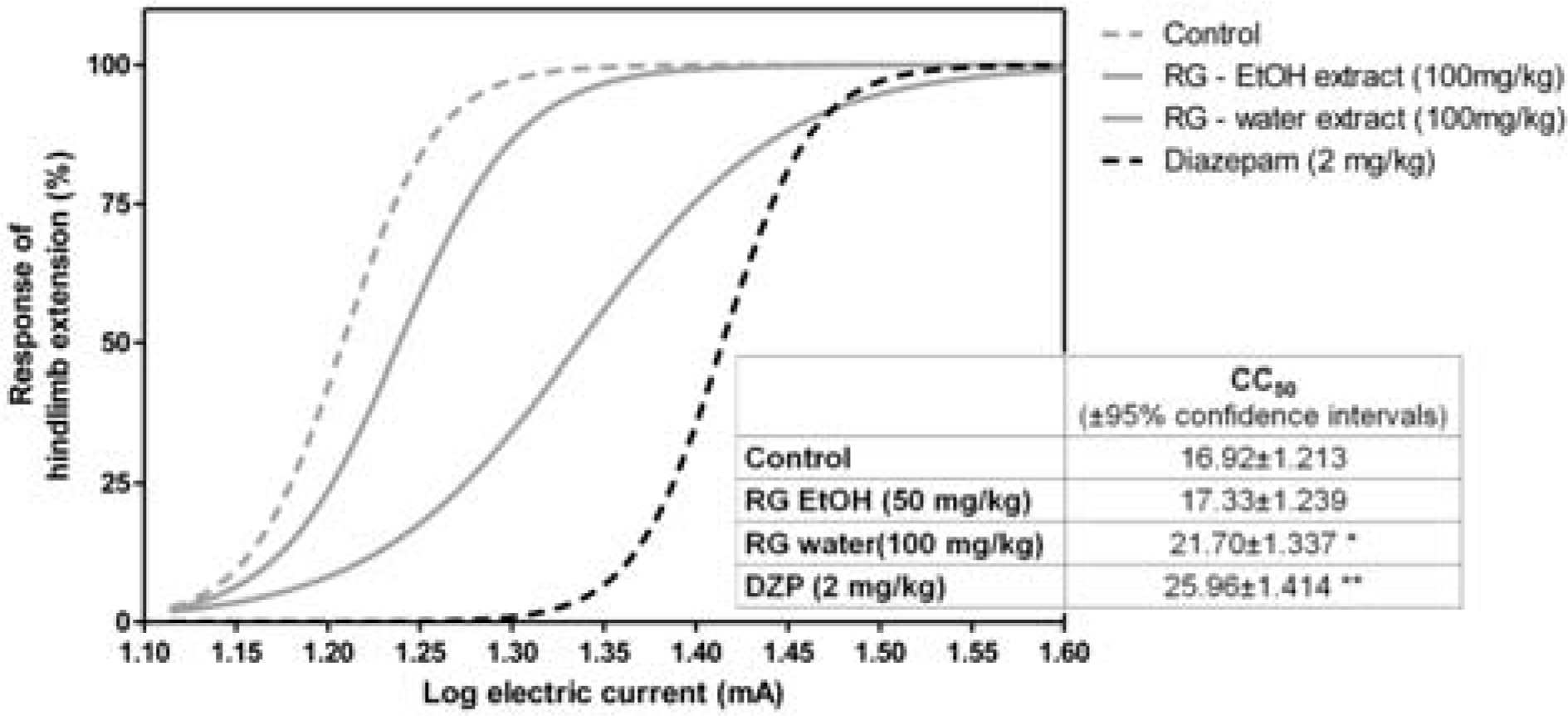 | Fig. 1.Effects of Rehmannia glutinosa (RG) water extract and ethanol extract on seizures induced by electroshock in animals (n = 10 per group). The numbers in the box represent CC50 ± 95% confidence intervals (∗p < 0.05 versus control; ∗∗p < 0.01 versus control; DZP, diazepam). |
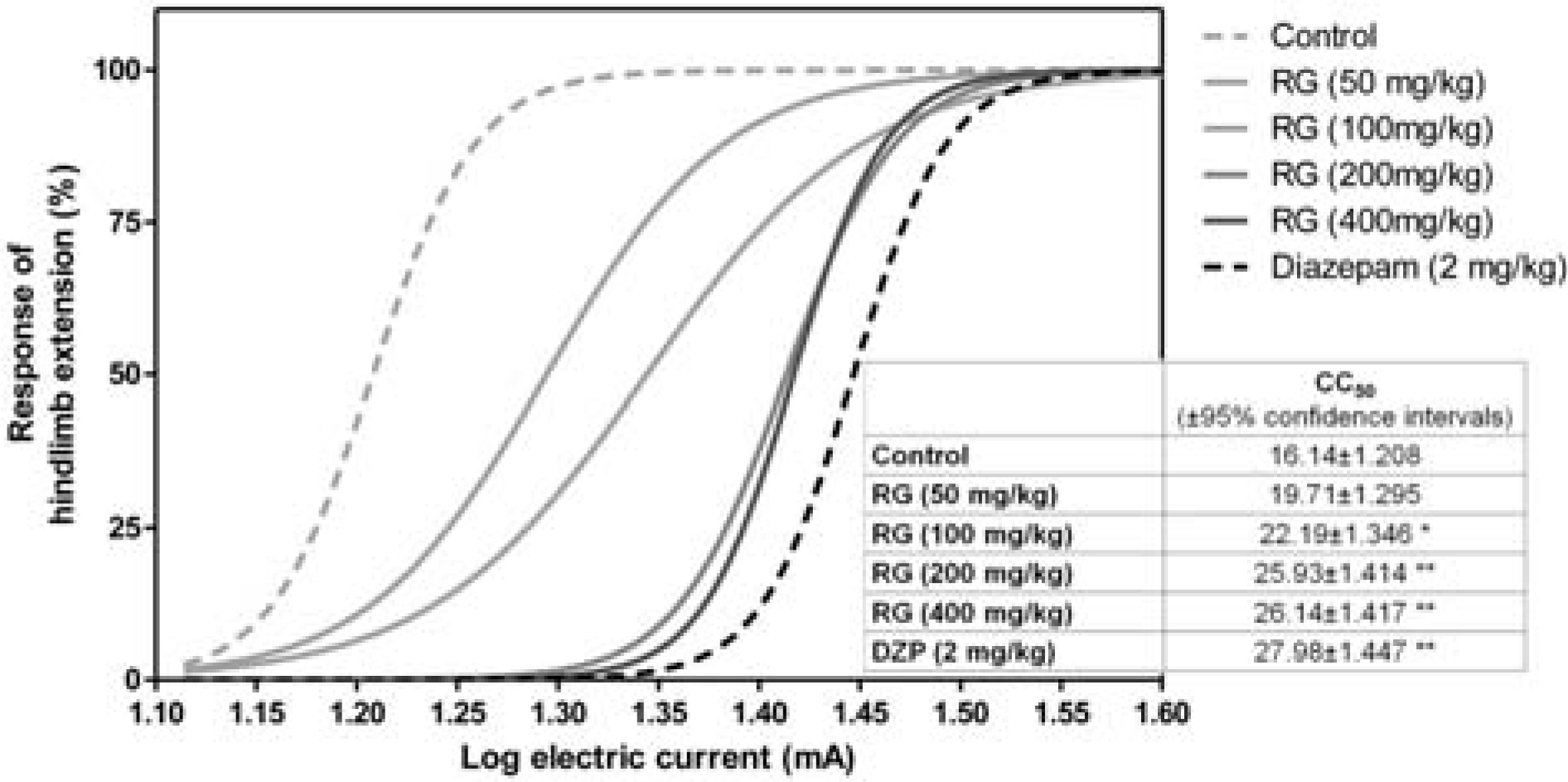 | Fig. 2.Effects of RG water extract on seizures induced by electroshock in animals (n = 20 per group). The numbers in the box represent CC50 ± 95% confidence intervals (∗p < 0.05 versus control; ∗∗p < 0.01 versus control; DZP, diazepam). |
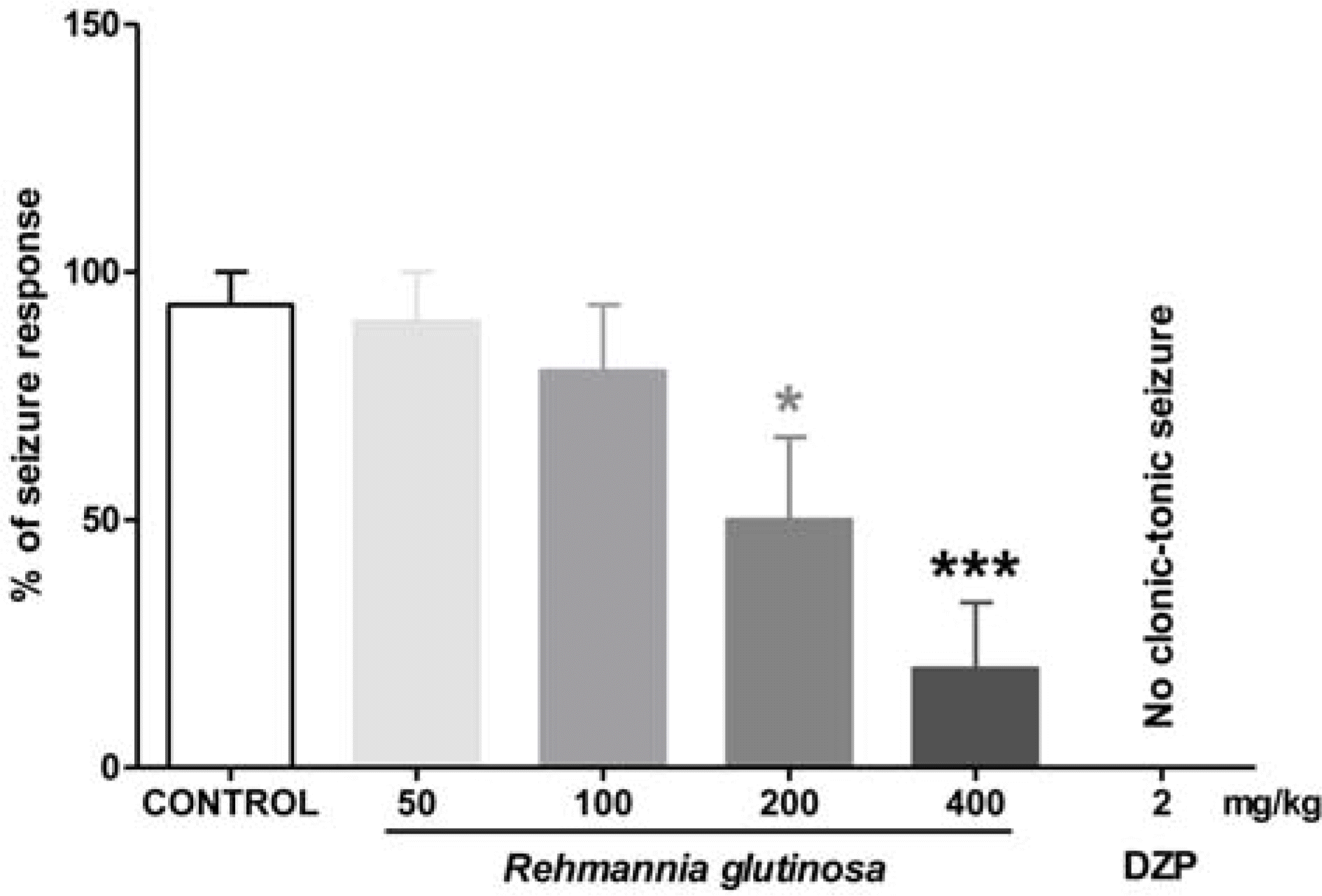 | Fig. 3.Effects of RG water extract on seizures induced by PTZ in animals (n = 15 per group). Each bar represents mean ± SEM of percent seizure response (∗p < 0.05 versus control; ∗∗∗p < 0.001 versus control; DZP, diazepam). |
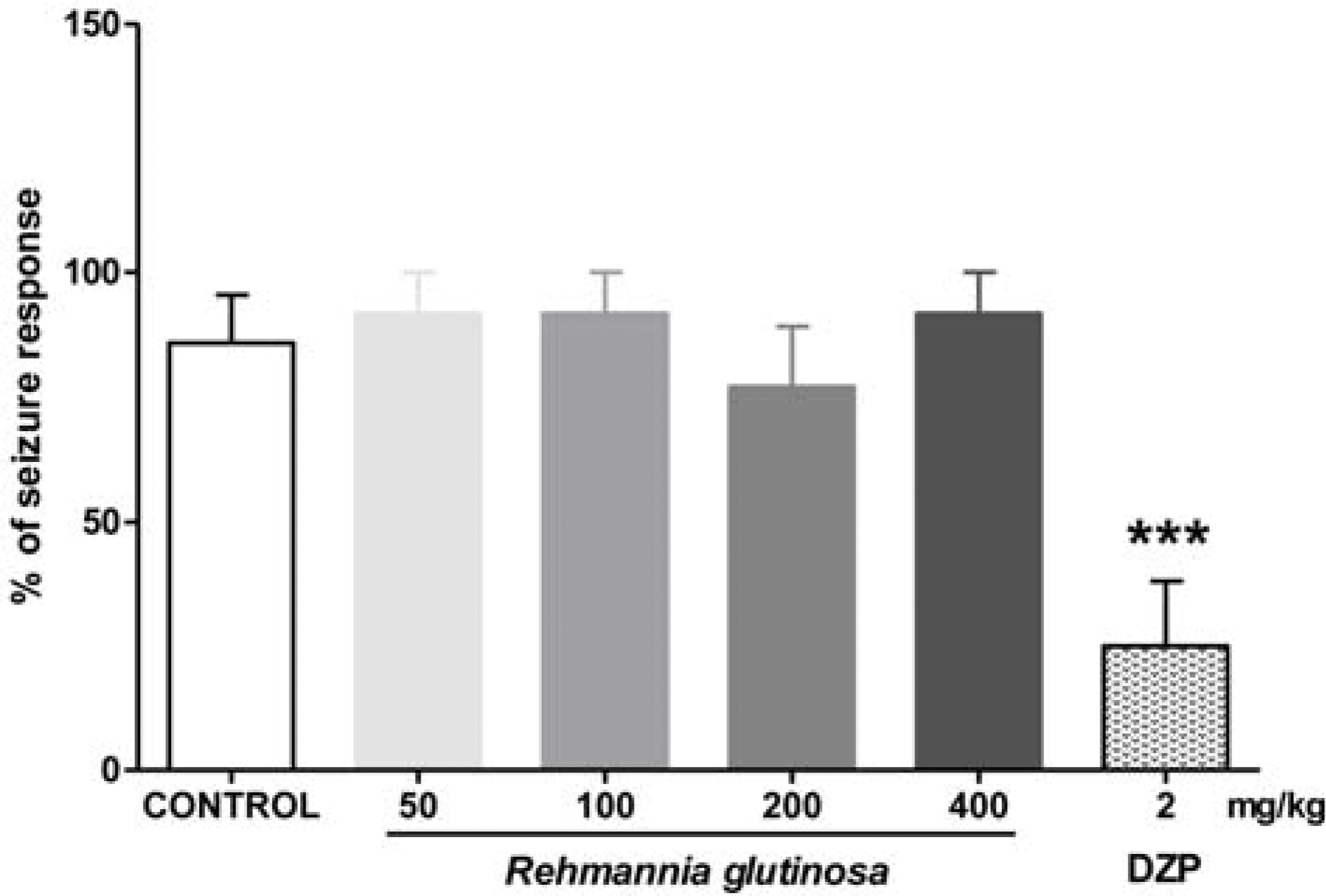 | Fig. 4.Effects of RG water extract on seizures induced by strychnine in animals (n = 12 − 14 per group). Each barrepresents mean ± SEM of percent seizure response (∗∗∗p < 0.001 versus control; DZP, diazepam). |
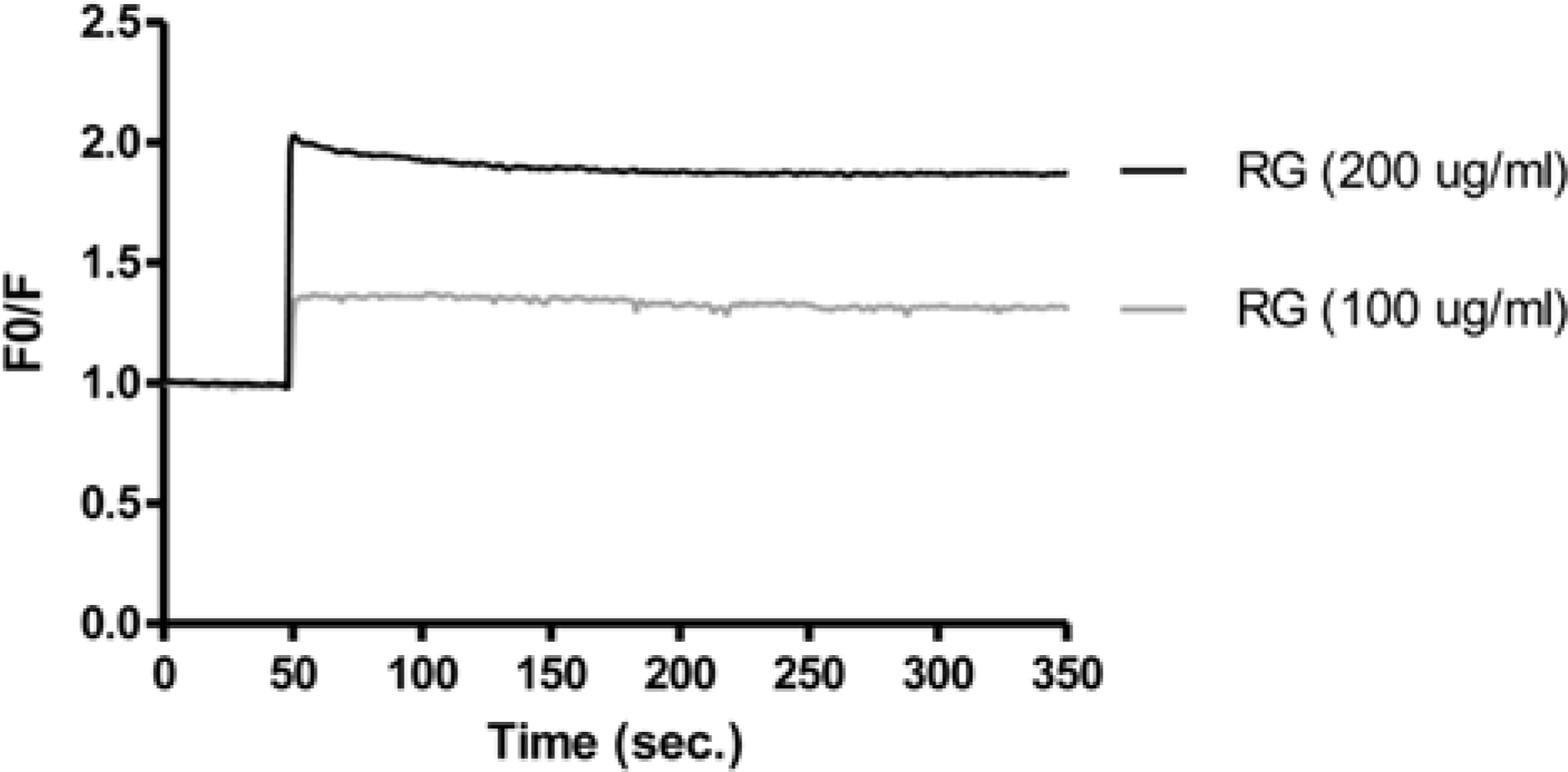 | Fig. 5.Effects of RG water extract on Cl− influx in neuroblastoma cells. Fluorescence was monitored with an excitation wavelength of 365 nm and an emission wavelength of 450 nm, using the Cl−-sensitive indicator, N-(methoxyquinolyl) acetoety-lester (MAQE). Contents of influx Cl− ion were expressed as a peak (a.u.). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download