Abstract
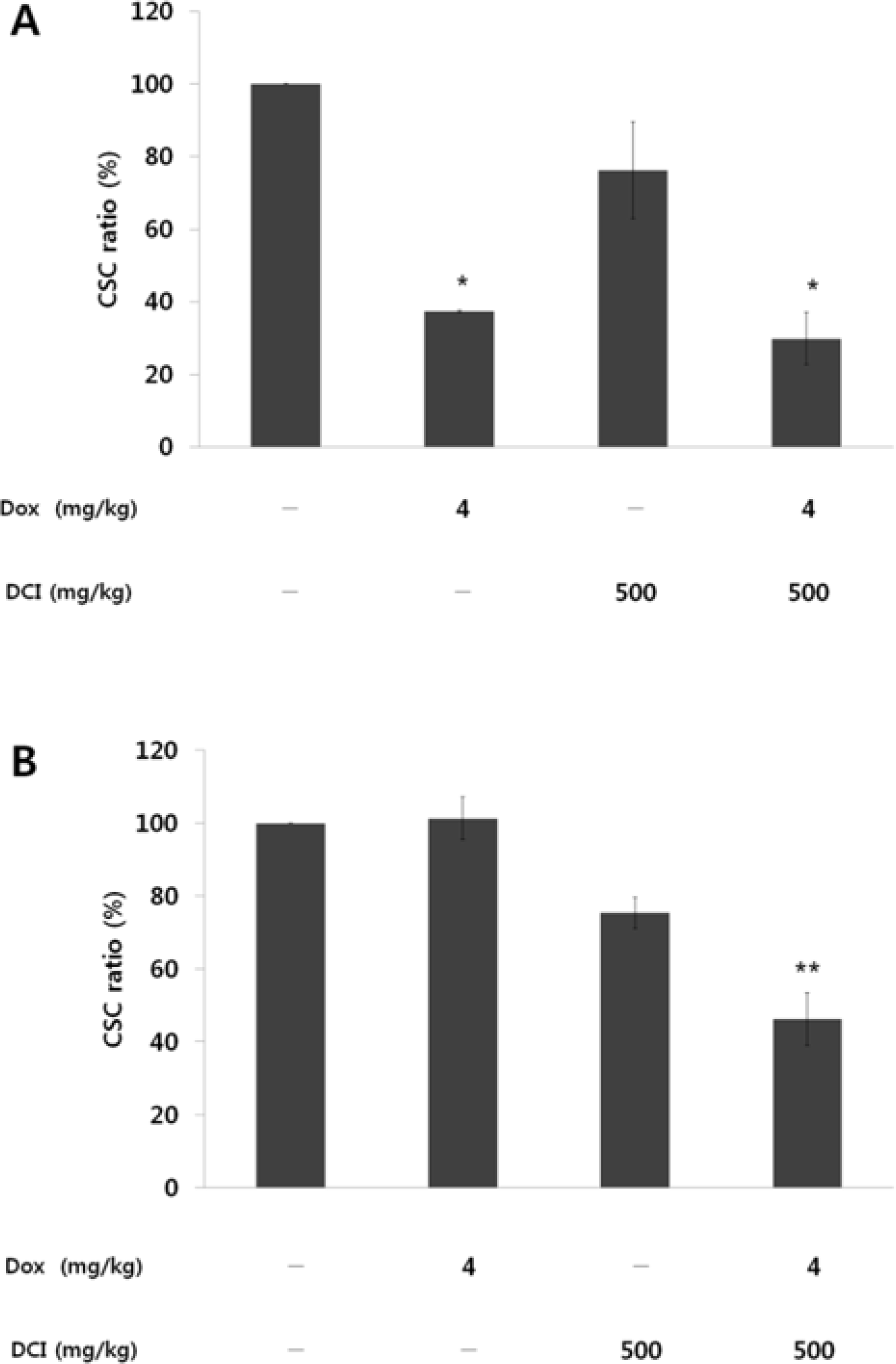
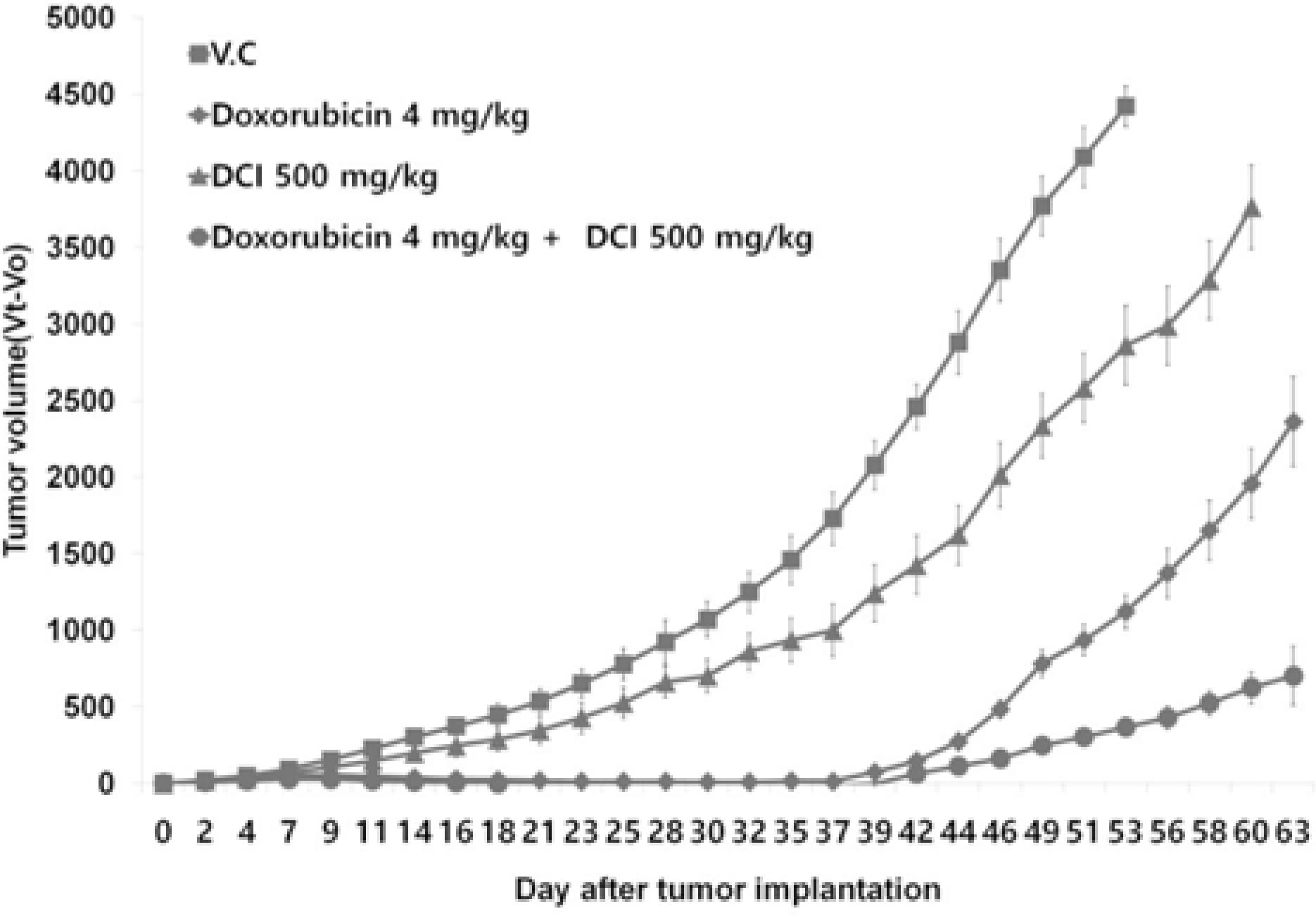
D-chiro-inositol (DCI) is a secondary messenger in insulin signal transduction. It is produced in vivo from myo-inositol via action of epimerase. In this study, we evaluated antitumor activity of DCI against human breast cancer both in vitro and in vivo. In order to determine the inhibitory effects of DCI on growth of human breast cancer cells (MDA-MB-231), two different assessment methods were implemented: MTT assay and mouse xenograft assay. MTT assay demonstrated downturn in cell proliferation by DCI treatment (1, 5, 10, 20 and 40 mM) groups by 18.3% (p < 0.05), 17.2% (p < 0.05), 17.5% (p < 0.05), 18.4% (p < 0.05), and 24.9% (p < 0.01), respectively. Also, inhibition of tumor growth was investigated in mouse xenograft model. DCI was administered orally at the dose of 500 mg/kg and 1000 mg/kg body weight to treat nude mouse for 45 consecutive days. On the 45th day, tumor growth of DCI (500 mg/kg and 1000 mg/kg) groups was suppressed by 22.1% and 67.6% as mean tumor volumes were 9313.8 ± 474.1 mm3 and 3879.1 ± 1044.1 mm3, respectively. Furthermore, breast cancer stem cell (CSC) phenotype (CD44+/CD24−) was measured using flow cytometry. On the 46th day, CSC ratios of DCI (500 mg/kg) and co-treatment with doxorubicin (4 mg/kg) and DCI (500 mg/kg) group decreased by 24.7% and 53.9% (p < 0.01), respectively. Finally, from tumor recurrence assay, delay of 5 days in the co-treatment group compared to doxorubicin (4 mg/kg) alone group was observed. Based on these findings, we propose that DCI holds potential as an anti-cancer drug for treatment of breast cancer.
Go to : 
References
(1). Larner J.Int. J. Exp. Diabetes Res. 2002. 3:47–60.
(2). Nestler J. E.., Jakubowicz D. J.., Reamer P.., Gunn R. D.., Allan G. N.Engl. J. Med. 1999. 340:1314–1320.
(3). Jung K. W.., Won Y. J.., Oh C. M.., Kong H. J.., Cho H.., Lee D. H.., Lee K. H.Cancer Res. Treat. 2015. 47:142–148.
(4). Jung K. W.., Won Y. J.., Kong H. J.., Oh C. M.., Cho H.., Lee D. H.., Lee K. H.Cancer Res. Treat. 2015. 47:127–141.
(5). Moody S. E.., Perez D.., Pan T. C.., Sarkisian C. J.., Portocarrero C. P.., Sterner C. J.., Notorfrancesco K. L.., Cardiff R. D.., Chodosh L. A.Cancer Cell. 2005. 8:197–209.
(6). Lacerda L.., Pusztai L.., Woodward W. A.Drug Resist. Updat. 2010. 13:99–108.
(7). McDermott S. P.., Wicha M. S.Mol. Oncol. 2010. 4:404–419.
(8). Dave B.., Mittal V.., Tan N. M.., Chang J. C.Breast Cancer Res. 2012. 14:202.
(9). Barkan D.., Chambers A. F.Clin. Cancer Res. 2011. 17:7219–7223.
(10). Reedijk M.., Pinnaduwage D.., Dickson B. C.., Mulligan A. M.., Zhang H.., Bull S. B.., O'Malley F. P.., Egan S. E.., Andrulis I. L.Breast Cancer Res. Treat. 2008. 111:439–448.
(11). Gangopadhyay S.., Nandy A.., Hor P.., Mukhopadhyay A.Clin. Breast Cancer. 2013. 13:7–15.
(12). Karamboulas C.., Ailles L.Biochim. Biophys. Acta. 2013. 1830:2481–2495.
(13). Izrailit J.., Reedijk M.Cancer Lett. 2012. 317:115–126.
(14). Hassounah N. B.., Bunch T. A.., McDermott K. M.Clin. Cancer Res. 2012. 18:2429–2435.
(15). Tang J.., Ahmad A.., Sarkar F. H.Int. J. Mol. Sci. 2012. 13:13414–13437.
(16). Li L.., Xie X.., Luo J.., Liu M.., Xi S.., Guo J.., Kong Y.., Wu M.., Gao J.., Xie Z.., Tang J.., Wang X.., Wei W.., Yang M.., Hung MC.., Xie X.Mol. Ther. 2012. 20:2326–2334.
(17). Sanguinetti A.., Bistoni G.., Avenia N. G.Chir. 2011. 32:438–446.
(18). Velasco-Velázquez M. A.., Homsi N.., De La Fuente M.., Pestell R. G.Int. J. Biochem. Cell Biol. 2012. 44:573–577.
Go to : 
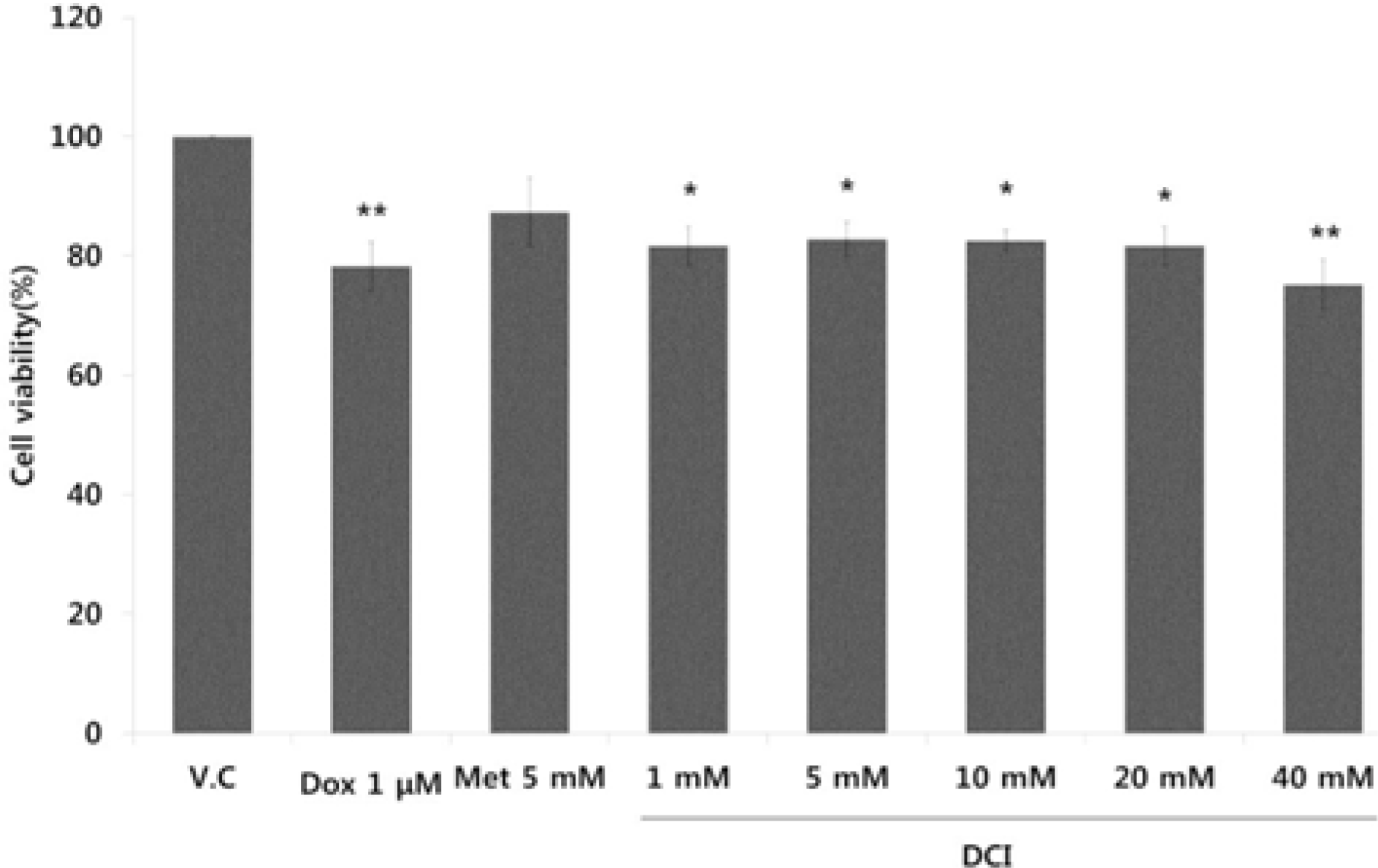 | Fig. 1.Growth inhibition of DCI on breast cancer cell lines. MDA-MB-231 cell lines were treated with 1 – 40 mM DCI for 48 hours. Doxorubicin (1 µM) and metformin (5 mM) were used as comparisons. Cell viability of MDA-MB-231 cell lines was estimated by MTT assay (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). |
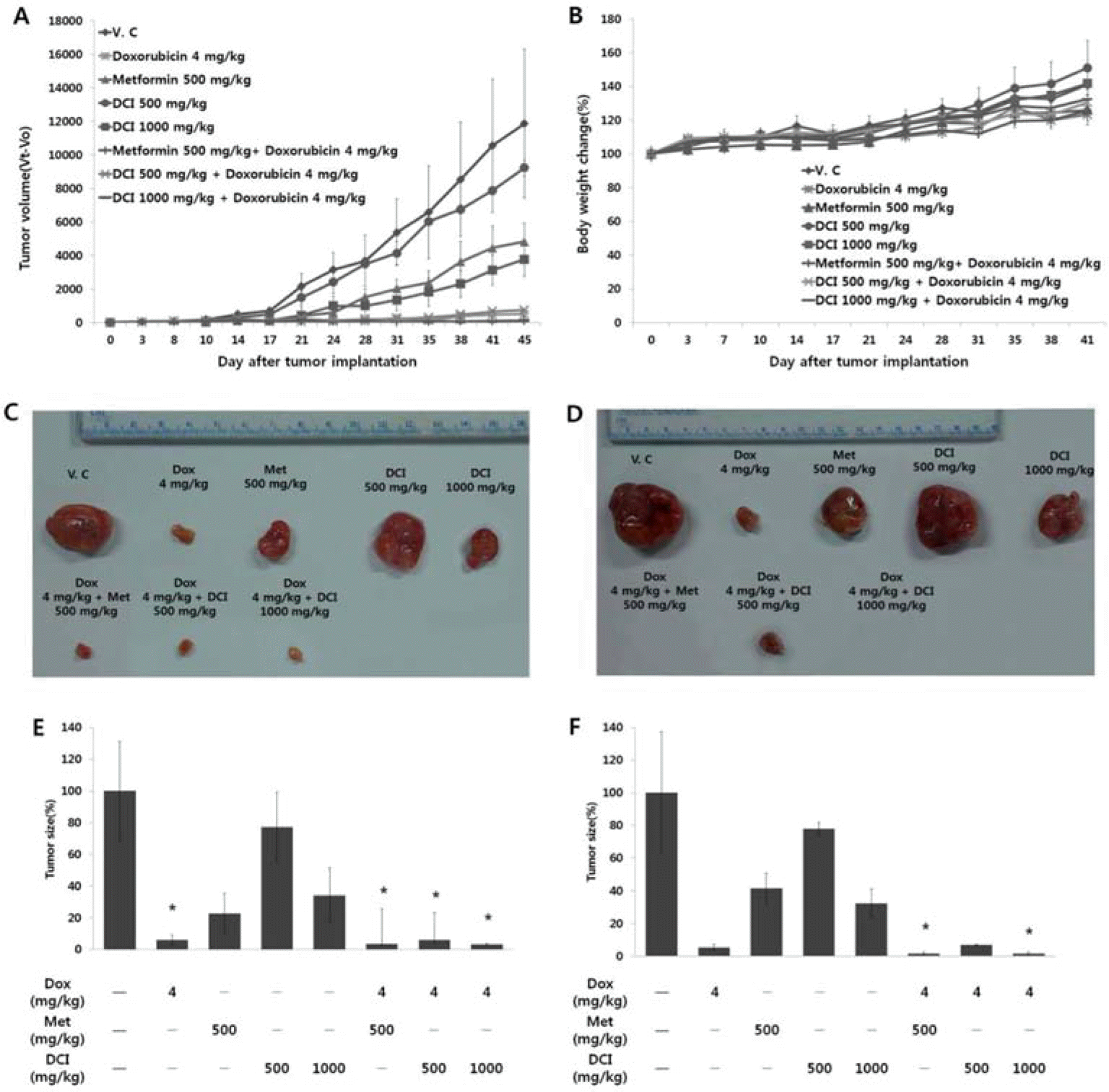 | Fig. 2.Inhibition of breast tumor growth by DCI in nude mouse xenograft assay. On day 0, nine million MDA-MB-231 cells were implanted subcutaneously into nude mice. DCI at a dose of 500 mg/kg or 1000 mg/kg body weight was administered orally for 45 consecutive days. (A) The body weights of the tumor-bearing nude mice were measured to evaluate overall toxicity of DCI. (B) Tumor volumes were estimated by the formula: length (mm) × width (mm) × height (mm)/2. Photographs of representative MDA-MB-231 tumor mass on (C) day 25 and (D) day 45 were shown. On (E) day 25 and (F) day 45, relative tumor size was measured (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download