Introduction
Experimental
General experimental procedures
Materials and microorganisms
Screening procedures
Scale-up fermentations of 6-DMAN and 5-O-PN
(±)-6-(1,1-Dimethylallyl)naringenin (1)
(±)-5-(O-Prenyl)naringenin-4′,7-diacetate (2)
(±)-8-Prenylnaringenin (3)
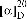 : 0° (c 0.028, MeOH); 1H-NMR (DMSO-d6, 300 MHz) δ 12.18 (1H, s, 5-OH), 10.78 (1H, s, 7-OH), 9.69 (1H, s, 4′-OH), 7.30 (2H, d, J = 8.5 Hz, H-2′/6′), 6.76 (2H, d, J = 8.5 Hz, H-3′/5′), 5.96 (1H, s, H-6), 5.42 (1H, dd, J = 2.8, 12.5 Hz, H-2), 5.08 (1H, t, J = 6.2 Hz, H-2″), 3.20 (1H, dd, J = 12.5, 16.3 Hz, H-3a), 3.08 (2H, d, J = 6.2 Hz, H-1″), 2.71 (1H, dd, J = 2.8, 16.3 Hz, H-3b), 1.58 (3H, s, H-4″), 1.53 (3H, s, H-5″); 13C NMR (DMSO-d6, 75 MHz) δ 196.7 (C-4), 164.3 (C-9), 161.1 (C-5), 159.7 (C-7), 157.5 (C-4′), 130.1 (C-3″), 129.2 (C-1′), 128.0 (C-2′/6′), 122.6 (C-2″), 115.1 (C-3′/5′), 106.9 (C-8), 101.7 (C-10), 95.2 (C-6), 78.2 (C-2), 41.9 (C-3), 25.5 (C-5″), 21.2 (C-1″), 17.6 (C-4″).
: 0° (c 0.028, MeOH); 1H-NMR (DMSO-d6, 300 MHz) δ 12.18 (1H, s, 5-OH), 10.78 (1H, s, 7-OH), 9.69 (1H, s, 4′-OH), 7.30 (2H, d, J = 8.5 Hz, H-2′/6′), 6.76 (2H, d, J = 8.5 Hz, H-3′/5′), 5.96 (1H, s, H-6), 5.42 (1H, dd, J = 2.8, 12.5 Hz, H-2), 5.08 (1H, t, J = 6.2 Hz, H-2″), 3.20 (1H, dd, J = 12.5, 16.3 Hz, H-3a), 3.08 (2H, d, J = 6.2 Hz, H-1″), 2.71 (1H, dd, J = 2.8, 16.3 Hz, H-3b), 1.58 (3H, s, H-4″), 1.53 (3H, s, H-5″); 13C NMR (DMSO-d6, 75 MHz) δ 196.7 (C-4), 164.3 (C-9), 161.1 (C-5), 159.7 (C-7), 157.5 (C-4′), 130.1 (C-3″), 129.2 (C-1′), 128.0 (C-2′/6′), 122.6 (C-2″), 115.1 (C-3′/5′), 106.9 (C-8), 101.7 (C-10), 95.2 (C-6), 78.2 (C-2), 41.9 (C-3), 25.5 (C-5″), 21.2 (C-1″), 17.6 (C-4″).(2S)-5,4′-Dihydroxy-7,8-[(R)-2-(1-hydroxy-1-methylethyl) -2,3-dihydrofurano]flavanone (4)
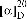 : −120° (c 0.056, MeOH); 1H-NMR (DMSO-d6, 300 MHz) δ 12.43 (1H, s, 5-OH), 9.69 (1H, s, 4′-OH), 7.32 (2H, d, J = 8.5 Hz, H-2′/6′), 6.80 (2H, d, J = 8.5 Hz, H-3′/5′), 5.96 (1H, s, H-6), 5.49 (1H, dd, J = 3.0, 12.2 Hz, H-2), 4.74 (1H, t, J = 8.5 Hz, H-2″), 3.26 (1H, dd, J = 12.2, 17.3 Hz, H-3a), 2.90 (2H, dd, J = 3.5, 8.5 Hz, H-1″), 2.72 (1H, dd, J = 3.0, 17.3 Hz, H-3b), 1.11 (3H, s, H-4″), 1.08 (3H, s, H-5″); 13C NMR (DMSO-d6, 75 MHz) δ 196.6 (C-4), 168.9 (C-7), 164.5 (C-5), 158.2 (C-4′), 157.4 (C-9), 129.2 (C-1′), 128.7 (C-2′/6′), 115.6 (C-3′/5′), 105.2 (C-8), 102.7 (C-10), 92.0 (C-2″), 90.9 (C-6), 78.9 (C-2), 70.4 (C-3″), 42.4 (C-3), 26.5 (C-1″), 26.1 (C-5″), 25.5 (C-4″).
: −120° (c 0.056, MeOH); 1H-NMR (DMSO-d6, 300 MHz) δ 12.43 (1H, s, 5-OH), 9.69 (1H, s, 4′-OH), 7.32 (2H, d, J = 8.5 Hz, H-2′/6′), 6.80 (2H, d, J = 8.5 Hz, H-3′/5′), 5.96 (1H, s, H-6), 5.49 (1H, dd, J = 3.0, 12.2 Hz, H-2), 4.74 (1H, t, J = 8.5 Hz, H-2″), 3.26 (1H, dd, J = 12.2, 17.3 Hz, H-3a), 2.90 (2H, dd, J = 3.5, 8.5 Hz, H-1″), 2.72 (1H, dd, J = 3.0, 17.3 Hz, H-3b), 1.11 (3H, s, H-4″), 1.08 (3H, s, H-5″); 13C NMR (DMSO-d6, 75 MHz) δ 196.6 (C-4), 168.9 (C-7), 164.5 (C-5), 158.2 (C-4′), 157.4 (C-9), 129.2 (C-1′), 128.7 (C-2′/6′), 115.6 (C-3′/5′), 105.2 (C-8), 102.7 (C-10), 92.0 (C-2″), 90.9 (C-6), 78.9 (C-2), 70.4 (C-3″), 42.4 (C-3), 26.5 (C-1″), 26.1 (C-5″), 25.5 (C-4″).(±)-5-(O-Prenylnaringenin)-4′-acetate (5)
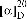 : 0° (c 0.052, MeOH); UV (MeOH) λmax: 286, 343 nm; IR (KBr) νmax cm−1: 3345, 1688, 1620, 1596, 1443, 1372, 1208; 1H-NMR (DMSO-d6, 300 MHz) δ 7.53 (2H, d, J = 8.5 Hz, H-2′/6′), 7.16 (2H, d, J = 8.5 Hz, H-3′/5′), 6.04 (1H, d, J = 1.6 Hz, H-6), 5.95 (1H, d, J = 1.6 Hz, H-8), 5.48 (1H, dd, J = 2.8, 12.5 Hz, H-2), 5.39 (1H, m, H-2″), 4.50 (2H, d, J = 6.2 Hz, H-1″), 2.97 (1H, dd, J = 12.5, 16.3 Hz, H-3a), 2.60 (1H, dd, J = 2.8, 16.3 Hz, H-3b), 2.27 (3H, s, -OAc), 1.74 (3H, s, H-4″), 1.69 (3H, s, H-5″); 13C NMR (DMSO-d6, 75 MHz) δ 187.0 (C-4), 169.2 (C-1‴), 165.6 (C-7), 164.0 (C-9), 161.3 (C-5), 150.3 (C-4′), 136.8 (C-3″), 136.4 (C-1′), 127.7 (C-2′/6′), 121.9 (C-3′/5′), 119.9 (C-2″), 104.2 (C-10), 95.8 (C-6), 94.9 (C-8), 77.4 (C-2), 65.1 (C-1″), 44.9 (C-3), 25.5 (C-4″), 20.9 (C-2‴), 18.1 (C-5″); HR-ESI-MS m/z 383.1494 [M+H]+ (calcd for C22H23O6, 383.1495).
: 0° (c 0.052, MeOH); UV (MeOH) λmax: 286, 343 nm; IR (KBr) νmax cm−1: 3345, 1688, 1620, 1596, 1443, 1372, 1208; 1H-NMR (DMSO-d6, 300 MHz) δ 7.53 (2H, d, J = 8.5 Hz, H-2′/6′), 7.16 (2H, d, J = 8.5 Hz, H-3′/5′), 6.04 (1H, d, J = 1.6 Hz, H-6), 5.95 (1H, d, J = 1.6 Hz, H-8), 5.48 (1H, dd, J = 2.8, 12.5 Hz, H-2), 5.39 (1H, m, H-2″), 4.50 (2H, d, J = 6.2 Hz, H-1″), 2.97 (1H, dd, J = 12.5, 16.3 Hz, H-3a), 2.60 (1H, dd, J = 2.8, 16.3 Hz, H-3b), 2.27 (3H, s, -OAc), 1.74 (3H, s, H-4″), 1.69 (3H, s, H-5″); 13C NMR (DMSO-d6, 75 MHz) δ 187.0 (C-4), 169.2 (C-1‴), 165.6 (C-7), 164.0 (C-9), 161.3 (C-5), 150.3 (C-4′), 136.8 (C-3″), 136.4 (C-1′), 127.7 (C-2′/6′), 121.9 (C-3′/5′), 119.9 (C-2″), 104.2 (C-10), 95.8 (C-6), 94.9 (C-8), 77.4 (C-2), 65.1 (C-1″), 44.9 (C-3), 25.5 (C-4″), 20.9 (C-2‴), 18.1 (C-5″); HR-ESI-MS m/z 383.1494 [M+H]+ (calcd for C22H23O6, 383.1495).(±)-Naringenin-4′-acetate (6)
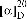 : 0° (c 0.024, MeOH); 1H-NMR (DMSO-d6, 300 MHz) δ 12.16 (1H, s, 5-OH), 7.55 (2H, d, J = 8.6 Hz, H-2′/6′), 7.19 (2H, d, J = 8.6 Hz, H-3′/5′), 5.87 (1H, d, J = 1.2 Hz, H-6), 5.84 (2H, d, J = 1.2 Hz, H-8), 5.56 (1H, dd, J = 2.7, 13.0 Hz, H-2), 3.26 (1H, dd, J = 13.0, 17.2 Hz, H-3a), 2.74 (1H, dd, J = 2.7, 17.2 Hz, H-3b), 2.29 (3H, s, -OAc).
: 0° (c 0.024, MeOH); 1H-NMR (DMSO-d6, 300 MHz) δ 12.16 (1H, s, 5-OH), 7.55 (2H, d, J = 8.6 Hz, H-2′/6′), 7.19 (2H, d, J = 8.6 Hz, H-3′/5′), 5.87 (1H, d, J = 1.2 Hz, H-6), 5.84 (2H, d, J = 1.2 Hz, H-8), 5.56 (1H, dd, J = 2.7, 13.0 Hz, H-2), 3.26 (1H, dd, J = 13.0, 17.2 Hz, H-3a), 2.74 (1H, dd, J = 2.7, 17.2 Hz, H-3b), 2.29 (3H, s, -OAc).(±)-Naringenin (7)
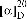 : 0° (c 0.021, MeOH); 1H-NMR (DMSO-d6, 300MHz) δ 12.17 (1H, s, 5-OH), 7.30 (2H, d, J = 8.5 Hz, H-2′/6′), 6.80 (2H, d, J = 8.5 Hz, H-3′/5′), 5.86 (2H, s, H-6/8), 5.41 (1H, dd, J = 2.8, 13.0 Hz, H-2), 3.25 (1H, dd, J = 13.0, 17.0 Hz, H-3a), 2.68 (1H, dd, J = 2.8, 17.0 Hz, H-3b).
: 0° (c 0.021, MeOH); 1H-NMR (DMSO-d6, 300MHz) δ 12.17 (1H, s, 5-OH), 7.30 (2H, d, J = 8.5 Hz, H-2′/6′), 6.80 (2H, d, J = 8.5 Hz, H-3′/5′), 5.86 (2H, s, H-6/8), 5.41 (1H, dd, J = 2.8, 13.0 Hz, H-2), 3.25 (1H, dd, J = 13.0, 17.0 Hz, H-3a), 2.68 (1H, dd, J = 2.8, 17.0 Hz, H-3b).Result and Discussion
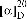 −120°).15 By comparison with the previously reported data,16 metabolite 4 was identified as (2S)-5,4′-dihydroxy-7,8-[(R)-2-(1-hydroxy-1-methylethyl)-2,3-dihydrofurano]flavanone.
−120°).15 By comparison with the previously reported data,16 metabolite 4 was identified as (2S)-5,4′-dihydroxy-7,8-[(R)-2-(1-hydroxy-1-methylethyl)-2,3-dihydrofurano]flavanone.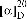 value of 5 was determined to be 0°, the same as rac-naringenin, indicated that 5 was also a racemate. On the basis of the spectral analyses and comparison with the spectral data of naringenin-4′,7-diacetate,13 the chemical structure of 5 was assigned (±)-5-(O-prenyl)naringenin-4′-acetate.
value of 5 was determined to be 0°, the same as rac-naringenin, indicated that 5 was also a racemate. On the basis of the spectral analyses and comparison with the spectral data of naringenin-4′,7-diacetate,13 the chemical structure of 5 was assigned (±)-5-(O-prenyl)naringenin-4′-acetate.



 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download