Abstract
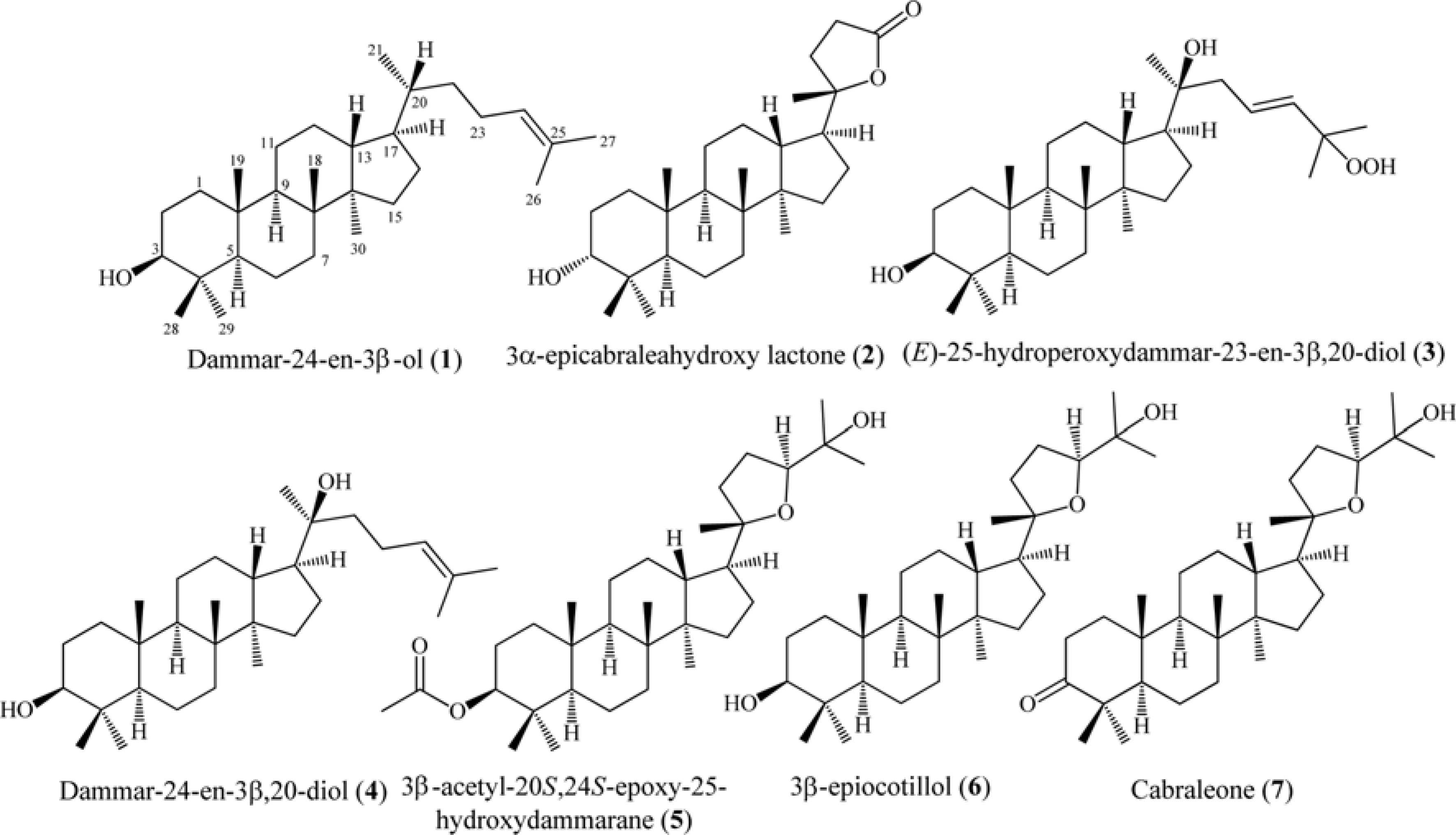
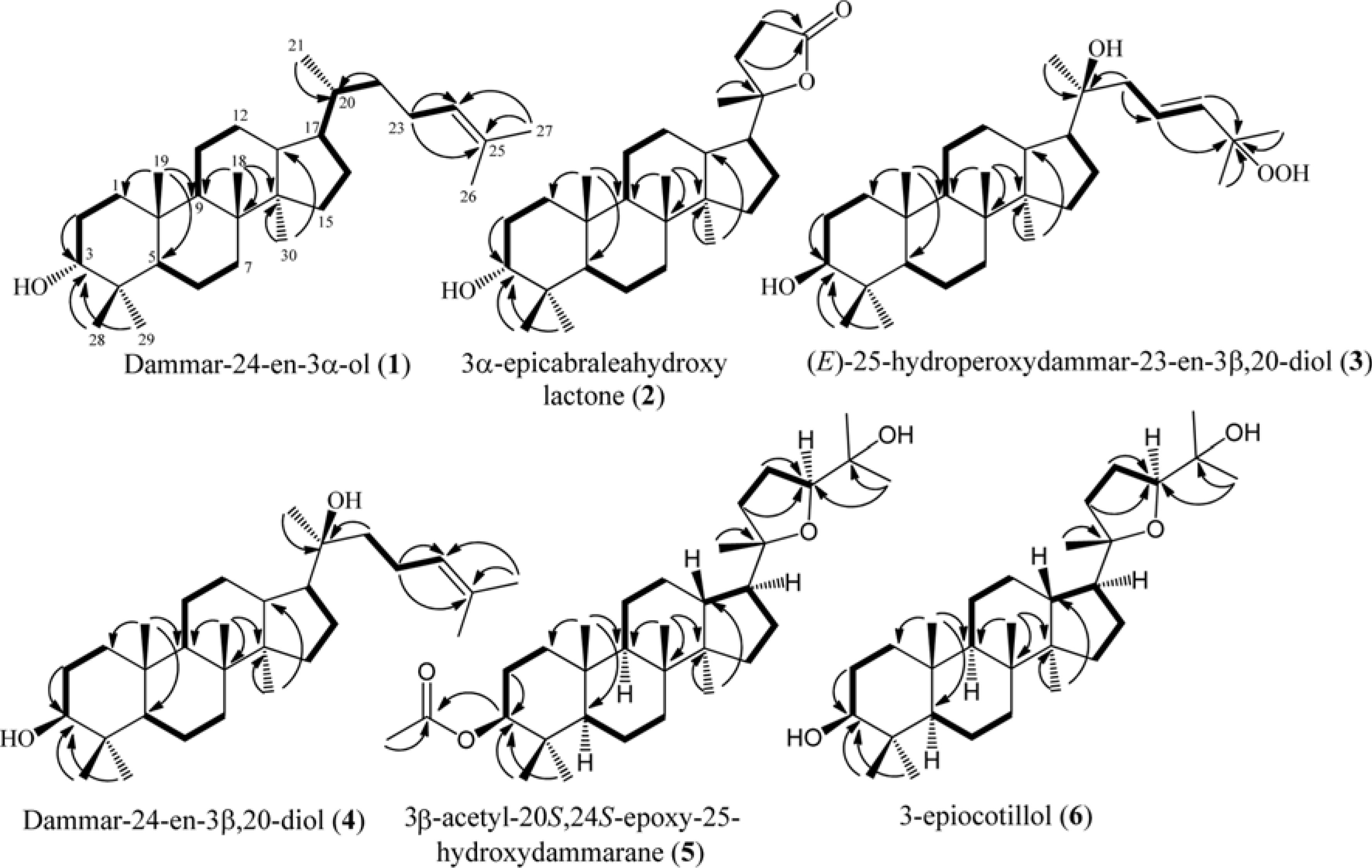
Six dammarane-type triterpenoids, dammar-24-en-3β-ol (1), 3β-epicabraleahydroxy lactone (2), (E)-25-hydroperoxydammar-23-en-3β,20-diol (3), dammar-24-en-3β,20-diol (4), 3β-acetyl-20S, 24S-epoxy-25-hydroxy-dammarane (5), and 3β-epiocotillol (6) were isolated from the methanolic extract of the bark of Aglaia elliptica. The chemical structure were identified on the basis of spectroscopic evidence and by comparison with those spectra previously reported. Compounds 1 – 6 were isolated first time from this plant. Compounds 1 – 6, along with a known synthetic analog, cabraleone (7) were evaluated their cytotoxic activity against P-388 murine leukimia cells in vitro. Among those compounds 3β-acetyl-20S, 24S-epoxy-25-hydroxydammarane (5) showed strongest cytotoxic activity with IC50 value of 8.02 ± 0.06 μM.
References
(1). Pannell C. M.Taxonomic monograph of the genus Aglaia lour (Meliaceae): Kew Bulletin Additional Series XVI; HMSO; Richmond,. 1992. 359–362.
(2). Harneti D.., Tjokronegoro R.., Safari A.., Supratman U.., Loong X.., Mukhtar M. R.., Mohamad K.., Awang K.., Hayashi H.Phytochem. Lett. 2012. 5:496–499.
(3). Muellner A. N.., Samuel R.., Chase M. W.., Pannell C. M.., Greger H.Am. J. Bot. 2005. 92:534–543.
(4). Cui B.., Chai H.., Santisuk T.., Reutrakul V.., Farnsworth N. R.., Cordell G. A.., Pezzuto J. M.., Kinghom A. D.Tetrahedron. 1997. 53:17625–17632.
(5). Ishibashi F.., Satasook C.., Ismant M. B.., Towers G. H. N.Phytochemistry. 1993. 32:307–310. http://www.sciencedirect.com/science/article/pii/S0031942200949860.
(6). Nugroho B. W.., Edrada R. A.., Wray V.., Witte L.., Bringmann G.., Gehling M.., Proksch P.Phytochemistry. 1999. 51:367–376.
(7). Wu T. S.., Liou M. J.., Kuoh C. S.., Teng C. M.., Nagao T.., Lee K. H. J.Nat. Prod. 1997. 60:606–608.
(8). Esimone C. O.., Eck G.., Nworu C. S.., Hoffmann D.., Uberla K.., Proksch P.Phytomedicine. 2010. 17:540–547.
(9). Sianturi J.., Purnamasari M.., Darwati ., Harneti D.., Mayanti T.., Supratman U.., Awang K.., Hayashi H.Phytochem. Lett. 2015. 13:297–301.
(10). Joycharat N.., Plodpai P.., Panthong K.., Yingyongnarongkul B.., Voravuthikunchai S. P.Can. J. Chem. 2010. 88:937–944.
(11). Liu S.., Liu S. B.., Zuo W. J.., Guo Z. K.., Mei W. L.., Dai H. F.Fitoterapia. 2014. 92:93–99.
(12). Yodsaoue O.., Sonprasit J.., Karalai C.., Ponglimanont C.., Tewtrakul S.., Chantrapromma S.Phytochemistry. 2012. 76:83–91.
(13). Cai X. H.., Wang Y. Y.., Zhao P. J.., Li Y.., Luo X. D.Phytochemistry. 2010. 71:1020–1024.
(14). Roux D.., Martin. M.., Adeline M.., Sevenet T.., Hadi A. H.., Pais M.Phytochemistry. 1998. 49:1745–1748.
(15). Xie B. J.., Yang S. P.., Chen H. D.., Yue J. M. J.Nat. Prod. 2007. 70:1532–1535.
(16). Zhang F.., Wang J. S.., Gu Y. C.., Kong L. Y. J.Nat. Prod. 2010. 73:2042–2046.
(17). Mohamad K.., Sévenet T.., Dumontet V.., Pa s M.., Tri M. V.., i Hadi H.., Awang K.., Martin M. T.Phytochemistry. 1999. 51:1031–1037.
(18). Awang K.., Loong X. M.., Leong K. H.., Supratman U.., Litaudon M.., Mukhtar M. R.., Mohamad K.Fitoterapia. 2012. 83:1391–1395.
(19). Mohamad K.., Martin M. T.., Najdar H.., Gaspard C.., Sevenet T.., Awang K.., Hadi H.., Pais M. J.Nat. Prod. 1999. 62:868–872.
(20). Farabi K.., Harneti D.., Nurlelasari; Maharani R.., Hidayat A. T.., Awang K.., Supratman U.., Shiono Y.Phytochem. Lett. 2017. 21:211–215.
(21). Breitmaier E.Structure elucidation by NMR in organic chemistry; John Wiley & Sons,. 2002. , London.
(22). Cysne Jde B.., Braz-Filho R.., Assuncao M. V.., Uchoa D. E.., Silveira E. R.., Pessoa O. D.Magn. Reson. Chem. 2006. 44:641–643.
(23). Phongmaykin J.., Kumamoto T.., Ishikawa T.., Suttisri R.., Saifah E.Arch. Pharm. Res. 2008. 31:21–27.
(24). Zhang F.., Wang J. S.., Gu Y. C.., Kong L. Y. J.Nat. Prod. 2010. 73:2042–2046.
(25). Sahidin ., Hakim E. H.., Juliawaty L. D.., Syah Y. M.., bin Din L.., Ghisalberti E. L.., Latip J.., Said I. M.., Achmad S. A. Z.Naturforsch. C. 2005. 60:723–727.
(26). Alley M. C.., Scudiero D. A.., Monks A.., Hursey M. L.., Czerwinski M. J.., Fine D. L.., Abbott B. J.., Mayo J. G.., Shoemaker R. H.., Boyd M. R.Cancer Res. 1988. 48:589–601.
(27). Hakim E. H.., Achmad S. A.., Juliawaty L. D.., Makmur L.., Syah Y. M.., Aimi A.., Kitajima M.., Takayama H.., Ghisalberti E. L. J.Nat. Med. 2007. 61:229.
Table 1.
13C-NMR data for compounds 1 – 7 (150 MHz in CDCl3)
Table 2.
Cytotoxicity activity of compounds 1 – 7 against P-388 murine leukemia cells
| Compounds | IC50 (µM) |
|---|---|
| Dammar-24-en-3α-ol (1) | 21.30 ± 0.06 |
| 3-epicabraleahydroxy lactone (2) | 104.71 ± 0.05 |
| (E)-25-hydroperoxydammar-23-en-3β,20-diol (3) | 12.41 ± 0.04 |
| Dammar-24-en-3β,20-diol (4) | 50.44 ± 0.04 |
| 3α-acetyl-20S, 24S-epoxy-25-hydroxydammarane (5) | 8.20 ± 0.06 |
| 3-epiocotillol (6) | 23.94 ± 0.04 |
| Cabraleone (7) | 32.86 ± 0.04 |
| Artonin E∗ | 0.68 ± 0.05 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download