Introduction
The dried ripe fruit of Zizyphus jujuba var. inermis and Zizyphus jujuba has been used in traditional Chinese medicine as an effective herbal remedy.
Various phytochemicals are present in the Zizyphus jujuba var. inermis and Zizyphus jujuba ranging from alkaloids, benzoids, cyclicpeptides, flavonoids, nucleosides, saponins, sesquiterpenoids, steroids, to triterpenoides. However, a full chemical analysis of the seed from Zizyphus jujuba var. inermis had not yet been performed.
In this study, we isolated and identified the structure of twenty two compounds (1 – 22) and observed anti-proliferative effect, antimicrobial activity, and anti-oxidant effect from the seeds of Z. jujuba var. inermis.
Experimental
General experimental procedures
The nuclear magnetic resonance (NMR) spectra data, including 1H-1H COSY, HMQC, HMBC and NOESY experiments, were obtained on a Bruker Biospin Avance II 400 MHz spectrometer. Fast Atom Bombardment Mass spectrometry (FAB-MS) and Liquid Chromatography (LC)-MSMS spectrometric data were acquired with a JMS-600W/JEOL, JMS-700 (JEOL) and AQUITY TQD (Waters) mass spectrometer. Silica Gel (230 – 400 mesh, Merck) was used for column chromatography. Thin Layer Chromatography (TLC) was carried out using Merck silica Gel 60 F254. HPLC (high performance liquid chromatography) was performed using a Waters 510 pump, Sodex RI71 detector [Porasil ™ Silica 1~20 µm, 19 mm ID × 300 mm] and Jasco pump PU-1580, UV 2075 plus [ODS (octadeoxysilane, C18) 5 µm, 10 mm ID × 250 mm (Daiso Co. Ltd.)]. HPLC solvents were from JT Baker, USA.
Materials
The dried red fruits of Zizyphus jujuba var. inermis were purchased from herbal market, Gyeongsan-si, Korea in October 2012. The materials were confirmed taxonomically by KGC raw materials headquarters, Korea Giseng Corp., Daejeon, Korea and voucher specimen ZJI-2012 was deposited at the R&D headquarters, Korea Ginseng Corp., Daejeon, Korea. The seeds were separated and collected from the dried red fruits of Z. jujuba var. inermis, the sarcocarp was washed off, and then the seeds were pulverized.
Extraction and isolation (Fig. 3)
The powder of the seeds (2 kg) were refluxed twice with water for 4 hours which yielded the water extract (719 g). The residue was refluxed twice with methanol and chloroform for 4 hours which yielded the methanol extract (70.8 g) and chloroform extract (4.2 g). The chloroform extract (4.2 g) was chromatographed on a silica gel column chromatography and eluted with a gradient of chloroform → chloroform: methanol = 50:1 → 10:1 → 9:1 → 8:1 → 7:1 → 6:1 → 5:1 → 1:1 → methanol in 2 fractions (C-1, C-2). Fraction C-1 (356 mg) was subjected to reverse phase HPLC eluting with a C18 ODS column (methanol-water = 95:5) to create 3-pentadecylcatechol (compound 1, 18 mg). The methanol extract (70.8 g) was chromatographed on a silica gel column chromatography and eluted with a gradient of n-hexane → n-hexane: ethyl acetate = 50:1 → 10:1 → 9:1 → 8:1 → 7:1 → 6:1 → 5:1 → 1:1 → ethyl acetate in 5 fractions (H-1, 2, 3, 4, 5). Fraction H-1 (2.76 g) was subjected to normal phase HPLC eluting with a silica gel column (n-hexane:ethyl acetate = 50:1) to create ρ-menth-3-ene (2, 23 mg), γ-bisabolene (3, 15 mg), vomifoliol (4, 18 mg). Fraction H-2 (13.2 g) was subjected to normal phase HPLC eluting with a silica gel column (n-hexane: ethyl acetate = 5:1) to create β-sitosterol (5, 28 mg), stigmasterol (6, 35 mg), stigmasta-5,23-dien-3β-ol (7, 15 mg), stigmast-4-en-3-one (8, 10 mg), lupeol (9, 12 mg), betulinic acid (10, 890 mg), betulinaldehyde (11, 56 mg), alphitolic acid (12, 12mg), 3-O-cis-ρ-coumaroyl-alphitolic acid (13, 8 mg), 3-O-trans-ρ-coumaroyl-alphitolic acid (14, 5 mg), 2-O-cis-ρ-coumaroyl-alphitolic acid (15, 17 mg), 2-O-trans-ρ-coumaroyl-alphitolic acid (16, 23 mg), zizyberanalic acid (17, 11 mg), ceanothic acid (18, 33 mg), oleanolic acid (19, 25 mg), maslinic acid (20, 26 mg), 3-O-cis-ρ-coumaroyl-maslinic acid (21, 13 mg), and 3-O-trans-ρ-coumaroyl-maslinic acid (22, 35 mg).
3-Pentadecylcatechol (1)
Yellow liquid, UV (MeOH) λmax (log ε) 280 (3.3), 220 (3.8) nm; FAB-MS m/z: 321.52 [M+H]+ (C21H37O2). This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.3
ρ-Menth-8-ene (2)
Yellow liquid, FAB-MS: m/z 139.25 [M+H]+ (C10H19). This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.8
γ-Bisabolene (3)
Yellow liquid, FAB-MS m/z: 205.36 [M+H]+ (C15H25). This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.9
Vomifoliol (4)
Yellow liquid, FAB-MS m/z: 225.30 [M+H]+ (C13H21O3). This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.10
Stigmasterol (6)
Amorphous powder; LC-MSMS (ES+) m/z: 413.71 [M+H]+ (C29H49O). This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.12
(23E)-Stigmasta-5,23-dien-3β-ol (7)
Amorphous powder; LC-MSMS (ES+) m/z: 413.72 [M+H]+ (C29H49O). This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.11
Stigmast-4-en-3-one (8)
Amorphous powder; LC-MSMS (ES+) m/z: 411.70 [M+H]+ (C29H47O). This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.1
Lupeol (9)
Amorphous powder; LC-MSMS (ES+) m/z: 427.73 [M+H]+ (C30H51O). This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.13
Betulinic acid (10)
Amorphous powder; LC-MSMS (ES+) m/z: 457.72 [M+H]+ (C30H49O3). This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.13
etulinaldehyde (11)
Amorphous powder; LC-MSMS (ES+) m/z: 441.72 [M+H]+ (C30H49O2). This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.14
Alphitolic acid (12)
Amorphous powder; LC-MSMS (ES+) m/z: 473.70 [M+H]+ (C30H49O4). This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.2
3-O-cis-ρ-Coumaroyl-alphitolic acid (13)
Amorphous powder; LC-MSMS (ES+) m/z: 619.86 [M+H]+ (C39H55O6). This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.2
3-O-trans-ρ-Coumaroyl-alphitolic acid (14)
Amorphous powder; LC-MSMS (ES+) m/z: 619.86 [M+H]+ (C39H55O6). This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.2
2-O-cis-ρ-Coumaroyl-alphitolic acid (15)
Amorphous powder; LC-MSMS (ES+) m/z: 619.87 [M+H]+ (C39H55O6). This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.2
2-O-trans-ρ-Coumaroyl-alphitolic acid (16)
Amorphous powder; LC-MSMS (ES+) m/z: 619.86 [M+H]+ (C39H55O6). This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.2
Zizyberanalic acid (17)
Amorphous powder; LC-MSMS (ES+) m/z: 471.40 [M+H]+.
This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.15
Ceanothic acid (18)
Amorphous powder; LC-MSMS (ES+) m/z: 487.70 [M+H]+ (C30H47O5). This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.16
Oleanolic acid (19)
Amorphous powder; LC-MSMS (ES+) m/z: 457.71 [M+H]+ (C30H49O3). This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.16
Maslinic acid (20)
Amorphous powder; LC-MSMS (ES+) m/z: 473.71 [M+H]+ (C30H49O4). This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.17
3-O-trans-ρ-Coumaroyl-maslinic acid (21)
Amorphous powder; LC-MSMS (ES+) m/z: 619.86 [M+H]+ (C39H55O6). This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.17
3-O-cis-ρ-Coumaroyl-maslinic acid (22)
Amorphous powder; LC-MSMS (ES+) m/z: 619.86 [M+H]+ (C39H55O6). This compound exhibited comparable spectroscopic data (1H and 13C-NMR) to published values.17
Cell proliferation assays
All cancer cell lines (K562, MEG-01, HL-60, KG-1, MOLT-4, L1210, P388D1, A549, HepG2, MCF-7, SK-OV-3, and SW-620) were maintained in RPMI 1640 which included 10% FBS and 1% penicillin/streptomycin. Cells were cultured at 37 in a 5% CO2 incubator. Each cell line was plated in 96-well plates at 1 × 104 cells/well. Cells were incubated with serial dilutions of each reagent for 48 hours. Cell proliferation was measured using the WST-1 assay with the Premix WST-1 cell proliferation assay system. In addition, IC50 values were calculated using CalcuSyn software (Biosoft, Cambridge, UK).
Evaluation of antioxidant effect
The antioxidant effect of the samples, based on the scavenging activity of the stable 2, 2′-diphenyl-1-picrylhydrazyl (DPPH) free radical, was determined by the method described by Blois, Mokbel and Hashinaga. The sample solutions (1.0 ml) in methanol at different concentrations were added to a 2 ml 0.004% (w/v) solution of DPPH in methanol, prepared daily and protected from light. The reaction mixture was incubated at room temperature in the dark for 30 min, and then the absorbance of the reactive mixture was recorded using spectrophotometer at 517 nm. Inhibition of the DPPH free radical in percent (I%) was calculated in following way: I%= [(Ablank−Asample)/Ablank] × 100; where Ablank is the absorbance of the control reaction (containing all reagents except the test compound), and Asample is the absorbance of the test compound. Compound concentration providing 50% inhibition (IC50) was calculated from the graph plotting inhibition percentage against solution concentration. Tests were carried out in triplicate. Six different concentrations of each sample studied have been assayed in order to check the linearity of response and to establish the antioxidant effect values in the adequate linear range. Methanol was tested against DPPH · radical and this resulted in null effect on the absorbance at 517 nm. Vitamin C, a known antioxidant, was used as positive control.
Antimicrobial activity
The samples showing activity against any of these were further tested against the following microorganisms: Staphylococcus aureus ATCC 6538™, Pseudomonas aeruginosa™ Escherichia coli ATCC 8739™, Candida albicans 10231™, and Aspergillus brasiliensis 16404™. The media used for testing the activity were Tryptic soy agar (236950, BD Difco™, USA). The infusion was done at 30℃ for 2–6 hours. The plates of microorganisms were incubated for 24 hours at 30℃. During assay, the fungal plates were incubated for 4–7 days at 30℃. The zone of inhibition of microbial growth was used as a measure of antimicrobial activity of the samples.
Result and Discussion
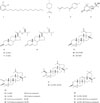
One urushiol, 3-pentadecylcatechol (1) was isolated for the first time from the seeds of Zizyphus jujuba var. inermis. The structure was identified through comparing of the FAB-MS, 1H- and 13C-NMR data with related data from the cited literature (Fig. 1).3 3-Pentadecylcatechol (1), which was isolated from Rhus vernicifera,3 is toxic, causing irritation, inflammation, and blistering of the skin,4 and has strong antioxidant,5 anticancer,6 and antimicrobial activity.7
One monoterpene, ρ-menth-8-ene (2) and one sesquiterpene, γ-bisabolene (3) were isolated for the first time from the seeds of Z. jujuba var. inermis. These structures were identified through comparing of the FAB-MS, 1H- and 13C-NMR data with related data from the cited literatures (Fig. 1).89 γ-Bisabolene (3) showed potent antiproliferative effect against human oral squamous cell carcinoma cells.21
Additionally, one sesquiterpene, vomifoliol (4),10 and four steroids: β-sitosterol (5),112 stigmasterol (6),112 stigmasta-5,23-dien-3β-ol (7),11 and stigmast-4-en-3-one (8),1 were isolated and identified (Fig. 1). Vomifoliol (4) showed significant antimicrobial activity against Neisseria gonorrhoeae.22 β-Sitosterol (5) showed antihyperlipidermic effect23 and antiproliferative effect against HL-60 cell line.24 Stigmasterol (6) showed remarkable antimicrobial activity against Streptococus mutans,25 Mycobacterium smegmatis, and M. aurum.28 β-Sitosterol (5) and stigma sterol (6) inhibit prostate cancer growth.26
Also, fourteen triterpenes: lupeol (9),13 betulinic acid (10),13 betulinaldehyde (11),14 alphitolic acid (12),2 3-O-cis-ρ-coumaroyl-alphitolic acid (13),2 3-O-trans-ρ-coumaroyl-alphitolic acid (14),2 2-O-cis-ρ-coumaroyl-alphitolic acid (15),2 2-O-trans-ρ-coumaroyl-alphitolic acid (16),2 zizyberanalic acid (17),15 ceanothic acid (18),16 oleanolic acid (19),16 maslinic acid (20),17 3-O-cis-ρ-coumaroyl-maslinic acid (21),17 and 3-O-trans-ρ-coumaroyl-maslinic acid (22),17 were isolated and identified as a by spectroscopic experiments, NMR and MS, and comparison with reported data (Fig. 1). Lupeol (9) showed antiproliferative effect against HELA-cell line.27 Lupeol (9) and betulinic acid (10) showed appreciable antimicrobial activity against M. smegmatis, M. aurum.28 Betulinic acid (10) showed potent anticancer activity.29 Betulinaldehyde (11) exhibited antimicrobial activity against M. tuberculosis.30 Alphitolic acid (12) exhibited antimicrobial activity against Staphylococcus aureus, Enterococcus faecalis, and Escherichia coli,31 and antiproliferative effect against oral squamous cell carcinoma cells.32 Betulinic acid (10), 3-O-cis-ρ-coumaroyl-alphitolic acid (13), and 3-O-trans-ρ-coumaroyl-alphitolic acid (14) showed high antiproliferative effects against K562, B-16, SK-MEL-2, PC-3, LOX-IMVI, and A-549 tumor cell.18 Ceanothic acid (18) exhibited antimicrobial activities against Streptococus mutans, Actinomyces viscosus, Porphyromonas gingivalis, and Prevotella intermedia.33 Maslinic acid (20) showed high antiproliferative effects against HT29 colon-cancer cell.34
3-Pentadecylcatechol (1), γ-bisabolene (3), β-sitosterol (5), stigmasterol (6), lupeol (9), betulinic acid (10), alphitolic acid (12), 3-O-cis-ρ-coumaroyl-alphitolic acid (13), 3-O-trans-ρ-coumaroyl-alphitolic acid (14) and maslinic acid (20) showed antiproliferative effects against HL-60, human oral squamous cell carcinoma, prostate cancer, HELA, K562, B-16, SK-MEL-2, PC-3, LOX-IMVI, A-549, and HT29 colon-cancer cells.7182124262729323334 The chloroform extract of seeds contains compounds (1, 3, 5, 6, 9, 10, 12, 13, 14 and 20), showed anti-proliferative effects with IC50 values of 100.5, 107.0, 68.0, and 15.2 µg/ml against K562, MOLT-4, L1210, and P388D1 cancer cell lines, respectively. But, the water extract was mostly inactive.
3-Pentadecylcatechol (1), vomifoliol (4), stigmasterol (6), lupeol (9), betulinic acid (10), betulinaldehyde (11), and alphitolic acid (12) showed for their antimicrobial activities against Actinomyces viscosus, Enterococcus faecalis, Escherichia coli, Mycobacterium smegmatis, M. aurum, Neisseria gonorrhoeae, Porphyromonas gingivalis, and Prevotella intermedia Staphylococcus aureus, Streptococus mutans.62225283031 The seeds extracts contain compounds (1, 4, 6, 9, 10, 11, 12, and 18) showed for their antimicrobial activities with IC50 values of water: methanol:chloroform = 760:210:110mg/mL against Staphylococcus aureus ATCC 6538™, Pseudomonas aeruginosa™ Escherichia coli ATCC 8739™, Candida albicans 10231™, and Aspergillus brasiliensis 16404™, respectively (Fig. 2). However, the sarcocarp of jujube did not show antimicrobial effects against Staphylococcus aureus.19
Pentadecylcatechol (1) has strong antioxidant activity20 Chemical constituents such as total phenolic are some of the most important materials of antioxidant activity from jujube.20 3-Pentadecylcatechol (1),5 3-O-cis-ρ-coumaroyl-alphitolic acid (13), 3-O-trans-ρ-coumaroyl-alphitolic acid (14), 2-O-cis-ρ-coumaroyl-alphitolic acid (15), 2-O-trans-ρ-coumaroyl-alphitolic acid (16), 3-O-cis-ρ-coumaroyl-maslinic acid (21), and 3-O-trans-ρ-coumaroyl-maslinic acid (22) are compounds containing a phenolic structure, there is a high possibility of exhibiting an antioxidative effect. The chloroform and methanol extracts of seed contain compounds (1, 13, 14, 15, 16, 21, and 22). The water, methanol, and chloroform extract of seeds were evaluated for antioxidant activity by scavenging of DPPH radical and showed IC50 values of 0.65, 0.32, and 3.27 mg/ml, respectively.
In this study, we investigated the component specificity of the jujube seed (Zizyphus jujuba var. inermis). Twenty-two compounds (1 – 22) were isolated from the extracts of seed. The structures were identified through comparing of the FAB-MS, 1H- and 13C-NMR data with related data from the cited literatures12389101112131617 : one urushiol (1), one monoterpenoid (2), two sesquiterpenoids (3 and 4), four steroids (5 – 8), and fourteen triterpenoids (9 – 22).
Compounds (1 – 3) were isolated and identified for the first time from Zizyphus jujuba var. inermis.
However, components such as alkaloids, cyclic peptides, flavones, nucleosides, and saponins found in fruit, cortex, root, etc. of Zizyphus jujuba var. inermis were not found in seeds.
Compounds (1, 3, 5, 6, 9, 10, 12, 13, 14 and 20) showed anti-proliferative effects,718212426272932333435 compounds (1, 4, 6, 9, 10, 11, 12, and 18) showed for their antimicrobial activities,62225283031 compounds (1, 13 – 16, and 21 – 22) showed antioxidant activity.3520 Similar to the data cited in the above literatures, each of the water, methanol, and chloroform extracts of the jujube seed showed a weak anti-proliferative effects, antimicrobial activity, and antioxidative effects, respectively.
Since the seed of jujube can cause allergies, it is instructed to roast seed in a folk medicine, heavy metals and proteins are being studied as the cause.35 However, as a result of this study on the components of the jujube seed, we could estimate that urushiol (1) was one of the cause of the allergy.
Furthermore, based on the results of the efficacy test and data cited in the literatures of compounds (1 – 22), it is confirmed that the jujube seed is an excellent food material having useful effects such as antioxidant, antibacterial, anticancer, anti-inflammatory, anti-diabetic, and antihyperlipidermic effect.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download