Abstract
The methylglyoxal (MGO) trapping constituents from onion (Allium cepa L.) peels were investigated using pre-column incubation of MGO and crude extract followed by HPLC analysis. The peak areas of MGO trapping compounds decreased, and their chemical structures were identified by HPLC-ESI/MS. Among major constituents in outer scale of onion, 2-(3,4-dihydroxybenzoyl)-2,4,6-trihydroxy-3(2H)-benzofuranone (2) was more effective MGO scavenger than quercetin (6) and its 4'-glucoside, spiraeoside (3). After 1 h incubation, compound 2 trapped over 90% MGO at a concentration of 0.5 mM under physiological conditions, but compounds 3 and 6 scavenged 45%, 16% MGO, respectively. HPLC-ESI/MS showed that compound 2 trapped two molecules of MGO to form a di-MGO adduct and compounds 3 and 6 captured one molecule of MGO to form mono-MGO adducts, and the positions 6 and 8 of the A ring of flavonoids were major active sites for trapping MGO.
Go to : 
References
(1). Ramasamy R.., Yan S. F.., Schmidt A. M.Cell. 2006. 124:258–260.
(2). Tang D.., Zhu J. X.., Wu A. G.., Xu Y. H.., Duan T. T.., Zheng Z. G.., Wang R. S.., Li D.., Zhu Q. J.Chromatogr. A. 2013. 1286:102–110.
(3). Lv L.., Shao X.., Chen H.., Ho C. T.., Sang S.Chem. Res. Toxico. 2011. 24:579–586.
(4). Lv L.., Shao X.., Wang L.., Huang D.., Ho C. T.., Sang S. J.Agric. Food Chem. 2010. 58:2239–2245.
(5). Schleicher E. D.., Wagner E.., Nerlich A. G. J.Clin. Invest. 1997. 99:457–468.
(6). Yoon S. R.., Shim S. M.LWT-Food Sci. Technol. 2015. 61:158–163.
(7). Shao X.., Bai N.., He K.., Ho C. T.., Yang C. S.., Sang S.Chem. Res. Toxicol. 2008. 21:2042–2050.
(8). Li X.., Zheng T.., Sang S.., Lv L. J.Agric. Food Chem. 2014. 62:12152–12158.
(9). Yang B. N.., Choi E. H.., Shim S. M.Appl. Biol. Chem. 2017. 60:57–62.
(10). Totlani V. M.., Peterson D. G. J.Agric. Food Chem. 2006. 54:7311–7318.
(11). Lo C. Y.., Li S.., Tan D.., Pan M. H.., Sang S.., Ho C. T.Mol. Nutr. Food Res. 2006. 50:1118–1128.
(12). Hu T. Y.., Liu C. L.., Chyau C. C.., Hu M. L. J.Agric. Food Chem. 2012. 60:8190–8196.
(13). Peng X.., Cheng K. W.., Ma J.., Chen B.., Ho C. T.., Lo C.., Chen F.., Wang M. J.Agric. Food Chem. 2008. 56:1907–1911.
(14). Chen X. Y.., Huang I. M.., Hwang L. S.., Ho C. T.., Li S.., Lo C. Y. J.Funct. Foods. 2014. 8:259–268.
(15). Zhu Y.., Zhao Y.., Wang P.., Ahmedna M.., Sang S.Chem. Res. Toxicol. 2015. 28:1842–1849.
(16). Ly T. N.., Hazama C.., Shimoyamada M.., Ando H.., Kato K.., Yamauchi R. J.Agric. Food Chem. 2005. 53:8183–8189.
(17). Xue Y. L.., Ahiko T.., Miyakawa T.., Amino H.., Hu F.., Furihata K.., Kita K.., Shirasawa T.., Sawano Y.., Tanokura M. J.Agric. Food Chem. 2011. 59:5927–5934.
(19). Shao X.., Chen H.., Zhu Y.., Sedighi R.., Ho C. T.., Sang S. J.Agric. Food Chem. 2014. 62:3202–3210.
(20). Wang P.., Chen H.., Sang S.Chem. Res. Toxicol. 2016. 29:406–414.
Go to : 
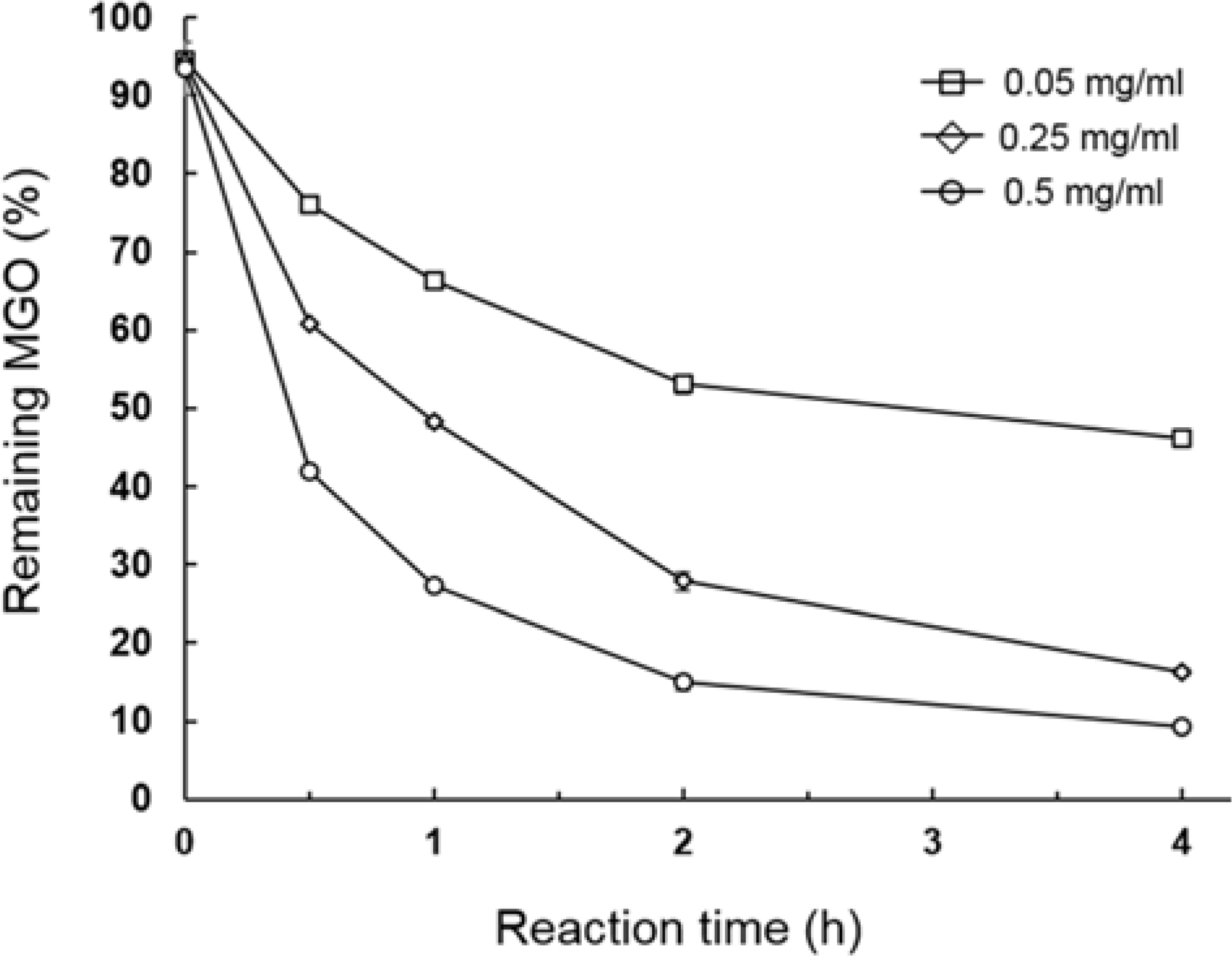 | Fig. 1.Scavenging of MGO by onion peel extracts (0.05, 0.25 and 0.5 mg/ml) under physiological conditions (pH 7.4, 37oC). Data are presented as the mean ± S.E. of three replications. |
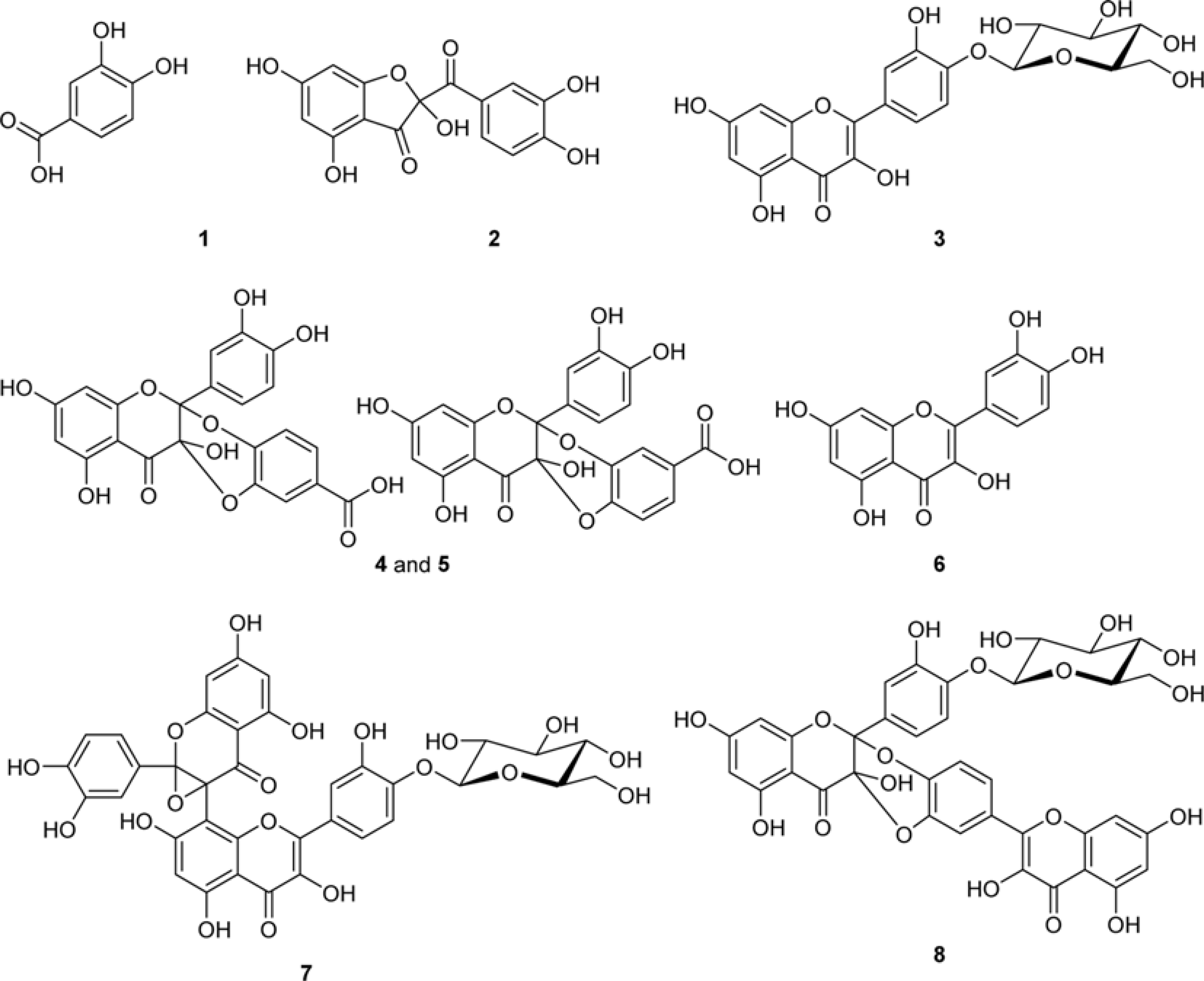 | Fig. 2.Chemical structures of the identified components from onion peel extract. The compounds No. are the same as in Fig. 3. and Table 1. |
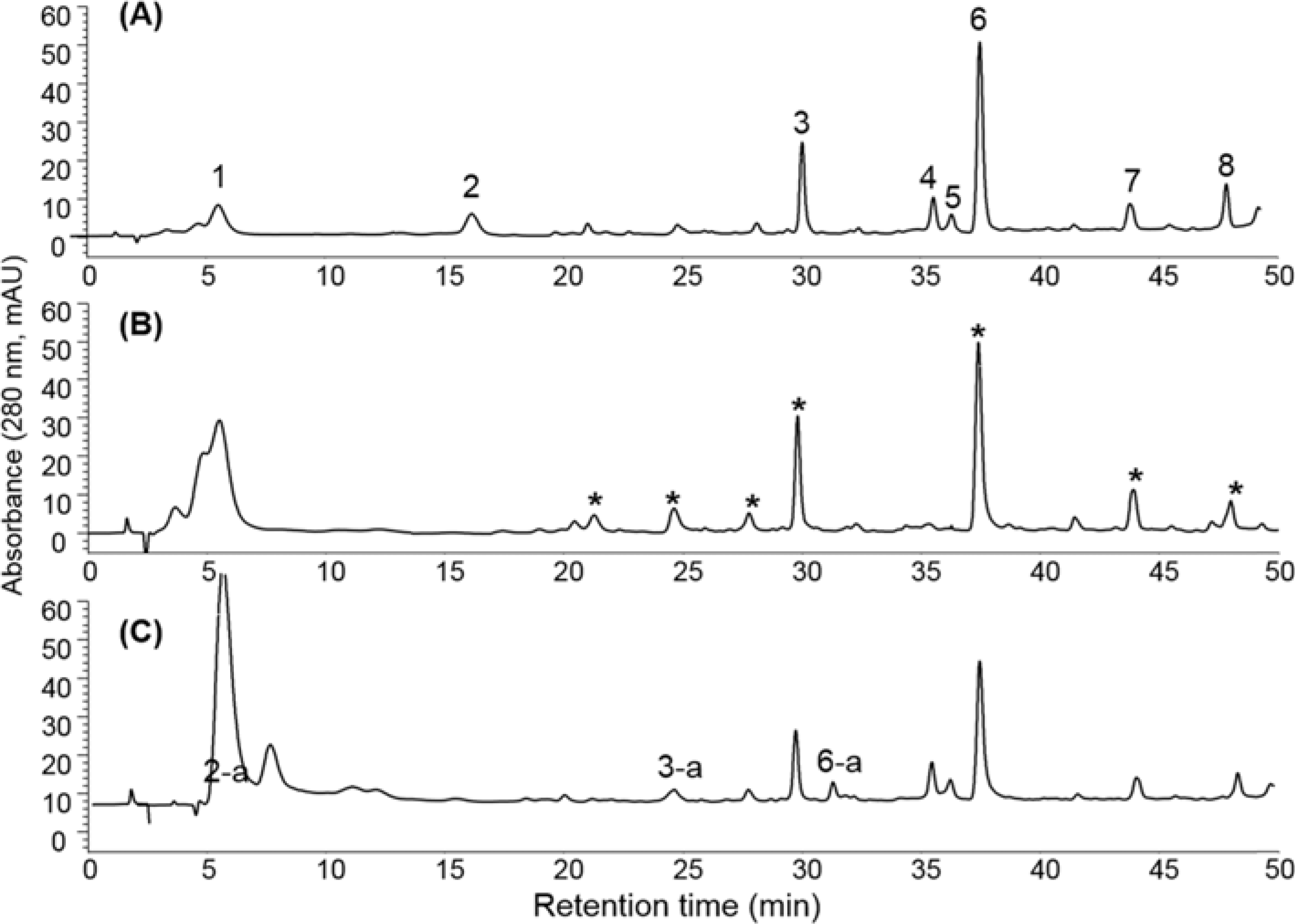 | Fig. 3.HPLC-DAD chromatograms of the onion peel extract. Onion peel extract dissolved in methanol (A), after incubation with PBS (100 mM, pH 7.4) for 12 h (B) and 10 mM MGO for 12 h, in PBS (C) at 37oC. The peaks designated as asterisk (∗) were potential MGO scavengers. Peaks 2-a, 3-a and 6-a appeared after reaction. |
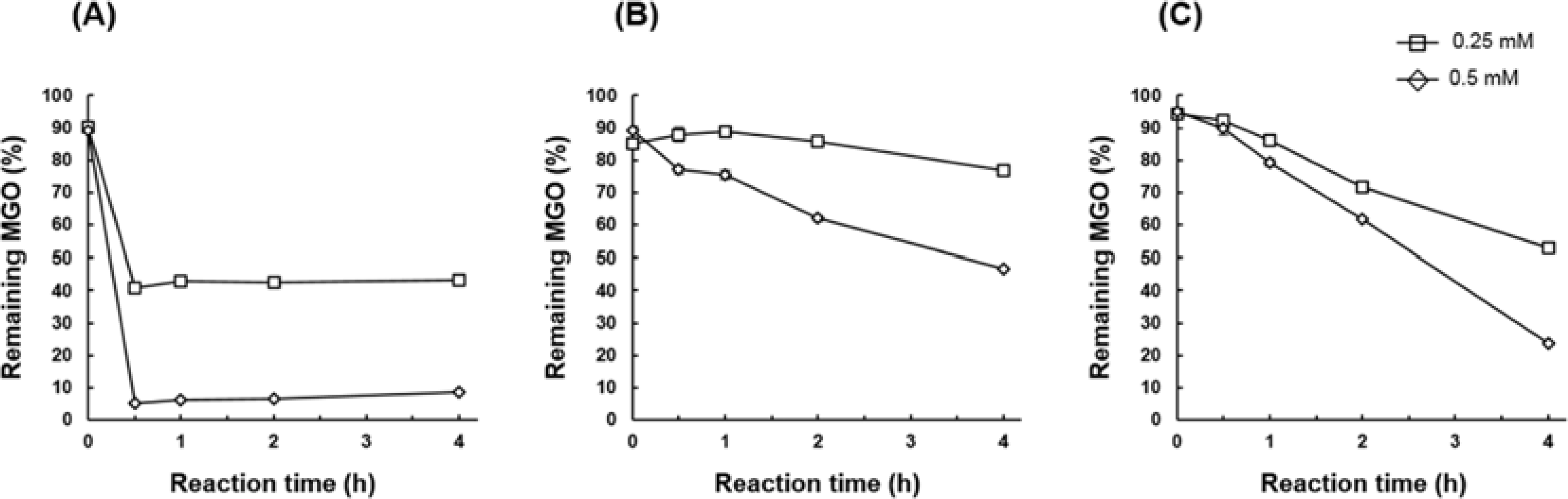 | Fig. 4.MGO scavenging capacity of isolated compounds 2, 3 and 6 under physiological conditions (pH 7.4, 37oC). Purified compounds 2, 3 and 6 (0.25 or 0.5 mM) were incubated with MGO (0.33 mM) for up to 4 h. (A) 2-(3,4-dihydroxybenzoyl)-2,4,6-trihydroxy-3(2H)-benzofuranone (2). (B) spiraeoside (3). (C) quercetin (6). |
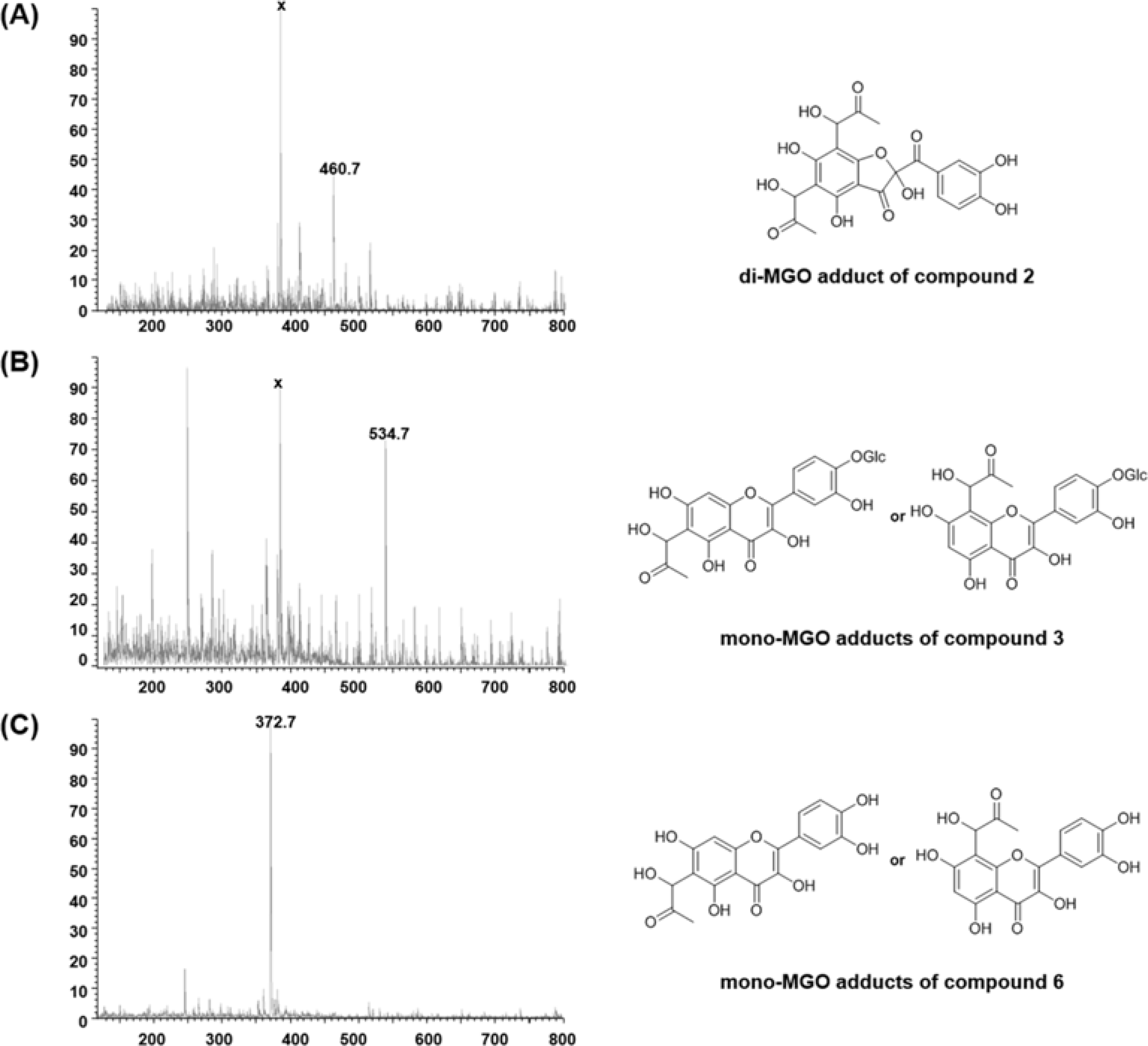 | Fig. 5.MS spectra of MGO adducts and proposed structures mono- or di-MGO adducts of compounds 2 (A), 3 (B) and 6 (C) under physiological conditions (pH 7.4, 37oC). |
Table 1.
Peak assignment for the components from onion peel extract




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download