Abstract
27-Hydroxycholesterol (27OHChol) has been reported to induce differentiation of monocytic cells into a mature dendritic cell phenotype. We examined the effect of methanol extract of Nardostachys chinensis (Nard) on 27OHChol-induced differentiation using THP-1, a human monocytic cell line. Treatment of monocytic cells with methanol extract of Nard resulted in decreased transcription and surface expression of CD80, CD83, and CD88 elevated by 27OHChol in a dose-dependent manner. Surface levels of MHC class I and II molecules elevated by 27OHChol were also reduced to basal levels by treatment with the Nard extract. Decreased endocytosis activity caused by 27OHChol was recovered by treatment with the Nard extract. CD197 expression and cell attachment were attenuated by the Nard extract. In addition, levels of transcription and surface expression of CD molecules involved in atherosclerosis, such as CD105, CD137, and CD166 upregulated by 27OHChol were significantly decreased by treatment with methanol extract of Nard. These results indicate that methanol extract of Nard down-regulates 27OHChol-induced differentiation of monocytic cells into a mature dendritic cell phenotype and expression of CD molecules associated with atherosclerosis. The current study suggests that biological activity of oxygenated cholesterol derivatives can be inhibited by herbal medication.
Monocytes can differentiate into dendritic cells (DCs) in response to multiple stimuli. Human blood CD14+ monocytes differentiate into DCs by culturing with granulocyte–macrophage colony stimulating factor (GM-CSF) and interleukin-4 (IL-4), and addition of tumor necrosis factor-α (TNF-α) promotes maturation into CD83high-DC.12 Blood monocytes differentiate into DC-like cells by treatment with lipopolysaccharide (LPS), TNF-α, and calcium ionophore.3 THP-1, a human monocytic cell line, also differentiates into DCs. Cytokines and ionomycin induce differentiation into mature DCs (mDCs),4 and oxygenated cholesterol molecules including 27-hydroxycholesterol (27OHChol) and 7-α hydroxycholesterol induce differentiation into mDC phenotype with high expression of CD80, CD83, CD86, and CD88.5 However, there is no information on herbal medicine that regulates 27OHChol-mediated differentiation into mDCs.
Nardostachys chinensis (Nard; the genus Nardostachys) is a Chinese medicinal herb mainly distributed in the Himalayas mountain areas. It is enriched with bioactive sesquiterpenes which exhibited anti-malarial and antinociceptive activities.6 The herb has been used as a traditional medicine for treatment of stomach-ache or inflammatory symptoms. Paste of Nard was reported to affect spontaneous ventricular arrhythmias in rats with acute myocardial infarction.7 Inhalation of dried Nard roots reduced the level of serum corticosterone, a stress-related hormone, increased by restraint stress causing stress cage.8 Methanol extract of Nard roots also inhibited lipopolysaccharides (LPS)-stimulated production of nitric oxide (NO) in RAW 264.7 macrophages.9 These studies suggest that Nard acts as an anti-oxidant and antiinflammatory reagent. However, the effects of Nardon immunological and inflammatory responses induced by cholesterol oxidation product are unknown.
We examined the effect of Nardon differentiation of DCs induced by 27OHChol. We report that 27OHChol-induced differentiation of monocytic cells is efficiently inhibited by methanol extract of Nard, as indicated by decreased expression of mDC markers and MHC molecules. In addition, we also examined whether the Nard extract could modulate expression of CD molecules which are associated with atherosclerosis.
THP-1 cells purchased from ATCC were maintained as previously described.5 27OHChol was purchased from Research Plus, Inc. (Bayonne, NJ, USA). Fluorescein isothiocyanate (FITC)-conjugated dextran (40 kDa) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Primary antibodies were purchased from Santa-Cruz Biotechnology (Santa Cruz, CA, USA). Alexa Fluor 488-conjugated secondary antibodies for FACS analysis were purchased from Invitrogen (Eugene, Oregon, USA).
The root of Nard was purchased from Hwalim Medicinal Herbs (Pusan, Korea). 50 g of dried Nard root was immersed in 500 ml of methanol and then sonicated for 15 min, followed by extraction of the samples for 24 h. Next, the supernatant was transferred and the sample was extracted in 500 ml of methanol for an additional 24 h. The extract was subsequently filtered through Whatman filter paper No. 20 and evaporated under reduced pressure using a vacuum evaporator (Eyela, Japan), followed by lyophilization of the condensed extract using a freeze dryer (Labconco, Kansas City, MO, USA). Finally, 4.41 g of lyophilized powder was obtained (yield, 8.82%). An aliquot of the extract was deposited at the Division of Pharmacology, School of Korean Medicine, Pusan National University (Voucher No. 2015-014).
Adherent cells were counted using Cell Counting Kit-8 (Dojindo Molecular Technologies, Inc. Rockville, MD, USA), following the manufacturer's instructions, as previously described (For more details, see the cited reference).5
Capacity of endocytosis after uptake of dextran-FITC conjugate was analyzed using a FACS Canto II (BD Biosciences, San Jose, CA, USA), as previously described(For more details, see the cited reference).5
Quantitative real-time PCR was performed using a Light-Cycler® 96 Real-Time PCR System (Roche, Germany), as previously described.10 The sequence of CD molecule primers was forward 5′-TGGTGCTGGCTGGTCTTTC and reverse 5′-CTGTGCCACTTCTTTCACTTCC (CD80); forward 5′-TCCTGAGCTGCGCCTACAG and reverse 5′-GCAG GGCAAGTCCACATCTT (CD83); forward 5′-GTGGTC CGGGAGGAGTACTTT and reverse 5′-GCCGTTTGTC GTGGCTGTA (CD88); forward 5′-CATCCTTGAAGTC CATGTCCTCTT and reverse 5′-GCCAGGTGCCATTTT GCTT (CD105); forward 5′-TCACTGCCTGGGGGCAG GAT and reverse 5′-GGCGGGGTCACAGAGGATGC (CD137); forward 5′-TCCTGCCGTCTGCTCTTCT and reverse 5′-TTCTGAGGTACGTCAAGTCGG (CD166). Primers for GAPDH were forward 5′-ATGGGGAAGGTGA AGGTCGand reverse 5′-GGGGTCAT TGATGGCAACAATA.
Expression of CD80, CD83, CD88, CD105, CD137, CD166, CD197, and major histocompatibility complex (MHC) class I and II molecules were analyzed using a FACS Canto II (BD Biosciences, San Jose, CA, USA), as previously described (For more information on antibodies, see the cited reference).5
Expression of mDC markers, like CD80, CD83 and CD88, was determined to assess the effect of Nardon differentiation of monocytic cells induced by 27OHChol (Fig. 1A). The level of CD80 was significantly increased to 8.15 fold, upon stimulation with 27OHChol, compared with unstimulated control. The increase was reduced to 7.02-, 5.14-, and 3.89-folds by treatment with 5, 25, and 50 µg/ml of methanol extract of Nard, respectively. The level of CD83 was increased to 11.76 fold by 27OHChol stimulation, which was reduced to 10.58-, 7.43-, and 4.19-folds by treatment with 5, 25, and 50 µg/ml of methanol extract of Nard, respectively. The level of CD88 increased to 21.98 fold by 27OHChol was reduced to 20.74-, 14.56-, and 9.28-folds by treatment with 5, 25, and 50 µg/ml of methanol extract of Nard, respectively. The inhibitory effects of methanol extract of Nard were not due to cytotoxicity because it did not affect viability of THP-1 cells (supplementary Fig. 1). The images of THP-1 cells treated with 27OHChol and methanol extract of Nard are shown in the supplementary Fig. 2.
Effects of Nardon surface expression of mDC markers was also examined (Fig. 1B). In agreement with results of real time-PCR, flow cytometric analyses showed increases of CD molecules upon stimulation with 27OHChol. The percentage of control cells positive for CD80 was 3.7%, which was increased to 24.7% by stimulation with 27OHChol. The increase was reduced to 21.3%, 10.2%, and 5.0%, respectively, by treatment with 5, 25, and 50 µg/ml of methanol extract of Nard. The percentage of CD83-positive control cells was 6.9%, which was increased to 26.5% by 27OHChol stimulation. The increase was reduced to 26.6%, 13.2%, and 9.2%, respectively, by treatment with 5, 25, and 50 µg/ml of methanol extract of Nard. The percentage of CD88-positive control cells was 8.9%, which was increased to 22.8% by 27OHChol. The increase was reduced to 20.8%, 12.0%, and 4.5%, respectively, by treatment with 5, 25, and 50 µg/ml of Nard extract. Collectively, the results indicate that Nard reduced not only transcription but also surface expression of mDC markers in monocytic cells.
MHC class molecules are highly expressed on mDCs, compared with monocyte or imDCs.11 The effect of Nardon expression of MHC class molecules was examined by flow cytometry (Fig. 2). The percentage of control cells positive for MHC class I molecule was 7.0%, which showed a significant increase to 15.3% after stimulation with 27OHChol, however, the increase was reduced to 12.4%, 6.8%, and 3.8% by treatment with 5, 25, and 50 µg/ml of methanol extract of Nard, respectively. Similarly, the percentage for MHC class II molecule was 4.9%, which was increased to 18.6% after 27OHChol stimulation. The increase was reduced to 13.6%, 14.0%, and 6.0% by treatment with 5, 25, and 50 µg/ml of Nard extract, respectively. These results indicate that Nard inhibited surface expression of MHC molecules.
Endocytosis activity is reduced on mDCs steps, compared with monocyte or imDCs steps.12 Endocytosis ability analyses were performed to determine the effect of Nardon functional alteration induced after stimulation with 27OHChol (Fig. 3). The percentage of control cells exhibiting endocytic activity was 18.2%, which was significantly reduced to 7.6% after stimulation with 27OHChol. The reduction was recovered to 8.5%, 16.2%, and 15.1% by treatment with 5, 25, and 50 µg/ml of Nard extract, respectively. This result indicates that endocytic function of monocytic cells changed by 27OHChol-stimulation was recovered by treatment with Nard extract.
CD197 is highly expressed on mDCs stage.12 The question of whether Nard affected expression of CD197 (CCR7), a homing molecule, was examined by flow cytometry (Fig. 4A). The percentage of control cells positive for CD197 was 3.4%, which was increased to 12.9% after stimulation with 27OHChol, however, the increase was reduced to 12.7%, 10.1%, and 5.1%, respectively, by treatment with 5, 25, and 50 µg/ml of methanol extract of Nard. We also investigated whether Nard affected the cell attachment (Fig. 4B). Following stimulation with 27OHChol attached cells were increased by 20.4 fold, but the attachment was reduced to 17.7-, 8.7-, and 4.3-folds, respectively, by treatment with 5, 25, and 50 µg/ml of Nard extract. These data show that treatment with Nard extract resulted in down-regulation of the morphological changes and over-expression of migration molecule induced by the oxidized lipid.
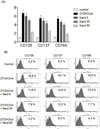
We examined whether Nard could influence expression of CD molecules CD105, CD137, and CD166, which are associated with atherosclerosis. Transcription of CD105, CD137, and CD166 was elevated, and addition of Nard resulted in decreased transcription of the genes (Fig. 5A). The level of CD105 was significantly increased to 6.67 fold by stimulation with 27OHChol, which was reduced to 5.96-, 4.74-, and 2.28-folds by treatment with 5, 25, and 50 µg/ml of methanol extract of Nard, respectively. The level of CD137 was increased to 5.48 fold by 27OHChol stimulation, which was reduced to 4.52-, 4.16-, and 2.52-folds by treatment with 5, 25, and 50 µg/ml of Nard extract, respectively. The CD166 level increased to 5.21fold by 27OHChol was reduced to 4.23-, 3.84-, and 1.76-folds by treatment with 5, 25, and 50 µg/ml of Nard extract, respectively.
Effects of Nardon surface expression of the molecules were also examined by flow cytometry (Fig. 5B). The percentage of control cells positive for CD105 was 4.2%, which showed a significant increase to 16.6% after stimulation with 27OHChol. However, the increase was reduced to 11.8%, 7.9%, and 4.2%, respectively, by treatment with 5, 25, and 50 µg/ml of methanol extract of Nard. The percentage of CD137-positive control cells was 6.2%, and it was increased to 19.1% by 27OHChol stimulation, which was reduced to 14.6%, 12.0%, and 8.0%, respectively, by treatment with 5, 25, and 50 µg/ml of Nard extract. The percentage of CD166-positive control cells was 6.2%, which was increased to 19.6% by 27OHChol, which was reduced to 13.2%, 7.7%, and 4.3%, respectively, by treatment with 5, 25, and 50 µg/ml of Nard extract. These results indicate that expression of CD105, CD137, and CD166 induced by 27OHChol was significantly inhibited by treatment with Nard extract at the levels of transcription and protein.
Previous studies demonstrated that over-activated T- and B-cells could promote atherosclerosis.1314 Specific markers for mDC over-expressed by 27OHChol are involved in activation of T- and B cells. CD80 (B7-1; CD28 ligand) is a surface receptor involved in B-cell activations.15 CD83 is a co-activator of T-cells and is required in development of CD4+ T cells.16 CD88 is a receptor for component C5a, an anaphylatoxin, and plays key roles in IL-12 secretion and in Th2 type immune response.17 Therefore, suppressed expression of the mDCs-specific markers indicates that extract of Nard will negative-regulate activation of T-/B-cells as well as differentiation of monocytic cells induced by 27OHChol. We assume that down-regulated expression of CD80, CD83, and CD88 by Nard can affect pathogenesis of atherosclerosis.
MHC molecules play an important role in presenting processed antigens captured by endo- or phagocytosis on the cell surface, and are highly expressed on mDCs in contrast to monocytes or immature DCs (imDCs).11 MHC molecules expressed in conjunction with the presented antigens stimulate naïve T-cells in secondary lymph nodes and induce adaptive immunity through various pathways.18 Our findings indicate that Nard extract can negatively regulate the expression of MHC molecules, thereby affecting antigen presentation. The reductions of MHC class I and II expression suggest that Nard can indirectly cause suppression of T-/B-cell activations through down-regulation of MHC class molecules on 27OHChol-exposed monocytic cells.
DCs digest and modify captured antigens taken up by endo- or phagocytosis in endo- or phagosome, and present using MHC molecules on the cell surface. DCs act as a different endocytosis activity in differentiation steps.1112 Endocytosis activity of DCs is powerful in immature but rare in mature steps. Nard-mediated recovery in endocytosis activity of monocytic cells suggests that Nard can regulate antigen uptake and its presentation on the cell surface because antigen presentation is affected by endocytosis activity. We assume that Nard-mediated recovery of endocytosis activity is associated with suppression of 27OHChol-induced differentiation. Taken together, these findings demonstrated that treatment with Nard resulted in recovery of changes of endocytic activity in monocytic cells differentiated by the oxysterol.
CD197, known as CCR7, is a homing molecule mainly expressing on mDCs, which plays an important role in migration of cells into secondary lymph nodes.12 A recent study showed that signals mediated by CCR7 are involved in immune responses including regulation of cell proliferation, activation, and differentiation of various cellular subpopulations.19 Therefore, our result indicates that Nard will affect migration and some immune responses mediated by CD197. Morphological change is an important characteristic in cell differentiation. The number of adherent cells was increased by treatment with 27OHChol, which is in agreement with our previous study,5 and treatment with methanol extract of Nard resulted in a dose-dependent suppression of the increase. Based on this observation, the differentiation induced by 27OHChol was negatively regulated by treatment with methanol extract of Nard.
CD105 (endoglin) is over-expressed in atherosclerotic tissues.2021 CD137 (4-1BB), which is also over-expressed in human atherosclerosis, promotes development of plaque inflammation,22 and its deficiency results in reduction of atherosclerosis induced by hypercholesterolemia.23 And CD166 is a marker for atherosclerosis (USA patent No.US8603829 B2). Therefore, the findings of the atherosclerosis-associated CD antigens by stimulation with 27OHChol, which is agreement with the previous study,5 indicate that 27OHChol is likely to increase their expression in atherosclerosis, thereby favoring development of the disease. In addition, decreased expression of the pro-atherosclerotic CD antigens will have positive effects by treatment with Nard extract because over-expression of the CD antigens is correlated with severity of atherosclerosis.
Taken together, we propose that Nard can inhibit differentiation of monocytic cells into mDC induced by 27OHChol, thereby down-regulating immune responses in a milieu rich in oxidatively modified cholesterol molecules like atherosclerotic plaques. The current study, however, was performed by using methanol extract of Nard. We think that the Nard extract is composed of various compounds, including sesquiterpenes, as analyzed by previous studies.68 Therefore, it is necessary to determine the active ingredients responsible for the inhibitory effects through further investigations.
Figures and Tables
Fig. 1
Effects of Nard extract on the transcription and surface expression of mDC-markers induced by 27OHChol. (A) THP-1 cells (1 × 106 cells/60 mm culture dish) were cultured for 48 h with 2.5 µg/ml of 27OHChol with or without 5, 25 or 50 µg/ml of Nard extract. Transcription of CD80, CD83, and CD88 was analyzed by real-time PCR. Data are expressed as the mean ± SD (n = 3 replicates/group). (B) THP-1 cells (1 × 106 cells/60 mm culture dish) were cultured for 48 h in the presence of 2.5 µg/ml of 27OHChol with or without 5, 25 or 50 µg/ml of Nard extract. Cells were immunostained with antibodies against CD80, CD83, and CD88 and analyzed by flow cytometry. Results are representative of three independent experiments.

Fig. 2
Effects of Nard extract on expression of MHC class I and II molecules induced by 27OHChol. Following incubation for 48 h with 27OHChol (2.5 µg/ml) with or without indicated concentrations of Nard extract, the stimulated THP-1 cells (1 × 106 cells/60 mm culture dish) were immunostained for MHC class I and II. Fluorescence was analyzed by flow cytometry. Results are representative of three independent experiments.

Fig. 3
Effects of Nard extract on functional alteration of monocytic cells induced by 27OHChol. Following incubation for 48 h with 27OHChol (2.5 µg/ml) with or without indicated concentrations of Nard extract, THP-1 cells were treated with 1 mg/ml of FITC-conjugated dextran for 1 h. The cells were analyzed by flow cytometry. Results are representative of three independent experiments.

Fig. 4
Effects of Nard extract on cell adhesion and expression of CD197 induced by 27OHChol. (A) THP-1 cells (1 × 106 cells/60 mm culture dish) were cultured for 48 h with 2.5 µg/ml of 27OHChol with or without indicated concentrations of Nard extract. The harvested cells were immunostained with an anti-CD197 antibody and analyzed by flow cytometry. Results are representative of three independent experiments. (B) THP-1 cells (2 × 104 cells/well of 96-well plate) were cultured for 48 h with 2.5 µg/ml of 27OHChol with or without indicated concentrations of Nard extract. Suspended cells in the well were removed, and the adherent cells were counted. Data are expressed as the mean ± SD (n = 3 replicates/group).
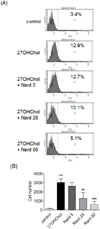
Fig. 5
Effects of Nard extraction expression of atherosclerosis-associated CD molecules induced by 27OHChol. (A) Following incubation with 27OHChol (2.5 µg/ml) for 48 h with or without indicated concentrations of Nard extract, transcription of CD105, CD137, and CD166 was analyzed by real-time PCR. Data are expressed as the mean ± SD (n = 3 replicates/group). (B) THP-1 cells (1 × 106 cells/60 mm culture dish) were cultured for 48 h with 2.5 µg/ml of 27OHChol with or without indicated concentrations of Nard extract. The harvested cells were immunostained with antibodies against CD105, CD137, and CD166 and analyzed by flow cytometry. Results are representative of three independent experiments.
References
1. Sallusto F, Lanzavecchia A. J Exp Med. 1994; 179:1109–1118.
2. Zhou LJ, Tedder TF. Proc Natl Acad Sci U S A. 1996; 93:2588–2592.
3. Lyakh LA, Koski GK, Telford W, Gress RE, Cohen PA, Rice NR. J Immunol. 2000; 165:3647–3655.
4. Berges C, Naujokat C, Tinapp S, Wieczorek H, Höh A, Sadeghi M, Opelz G, Daniel V. Biochem Biophys Res Commun. 2005; 333:896–907.
5. Son Y, Kim SM, Lee SA, Eo SK, Kim K. Biochem Biophys Res Commun. 2013; 438:161–168.
6. Takaya Y, Takeuji Y, Akasaka M, Nakagawasai O, Tadano T, Kisara K, Kim K, Wataya Y, Niwa M, Oshima Y. Tetrahedron. 2000; 56:7673–7678.
7. Zhang J, Qiang CC, Li WJ, Liu LJ, Lin XX, Cheng YJ, Tang K, Yao FJ, Wu SH. J Cardiovasc Pharmacol. 2014; 64:127–133.
8. Takemoto H, Omameuda Y, Ito M, Fukuda T, Kaneko S, Akaike A, Kobayashi Y. Biol Pharm Bull. 2014; 37:1050–1055.
9. Hwang JS, Lee SA, Hong SS, Han XH, Lee C, Lee D, Lee CK, Hong JT, Kim Y, Lee MK, Hwang BY. Bioorg Med Chem Lett. 2012; 22:706–708.
10. Seo HC, Kim SM, Eo SK, Rhim BY, Kim K. Biomol Ther (Seoul). 2015; 23:84–89.
11. Steinman RM. Annu Rev Immunol. 1991; 9:271–296.
12. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Annu Rev Immunol. 2000; 18:767–811.
13. Perry HM, McNamara CA. Arterioscler Thromb Vasc Biol. 2012; 32:1548–1549.
14. Profumo E, Buttari B, Saso L, Capoano R, Salvati B, Riganò R. Scientific World J. 2012; 2012:157534.
15. Katz FE, Parkar M, Stanley K, Murray LJ, Clark EA, Greaves MF. Eur J Immunol. 1985; 15:103–106.
16. Fujimoto Y, Tu L, Miller AS, Bock C, Fujimoto M, Doyle C, Steeber DA, Tedder TF. Cell. 2002; 108:755–767.
17. Gavett SH, O'Hearn DJ, Li X, Huang SK, Finkelman FD, Wills-Karp M. J Exp Med. 1995; 182:1527–1536.
18. Banchereau J, Steinman RM. Nature. 1998; 392:245–252.
19. Moschovakis GL, Bubke A, Dittrich-Breiholz O, Braun A, Prinz I, Kremmer E, Förster R. Eur J Immunol. 2012; 42:48–57.
20. DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. Mol Endocrinol. 2008; 22:65–77.
21. Piao M, Tokunaga O. J Atheroscler Thromb. 2006; 13:82–89.
22. Olofsson PS, Söderström LA, Wågsäter D, Sheikine Y, Ocaya P, Lang F, Rabu C, Chen L, Rudling M, Aukrust P, Hedin U, Paulsson-Berne G, Sirsjö A, Hansson GK. Circulation. 2008; 117:1292–1301.
23. Kim SM, Kim BY, Lee SA, Eo SK, Yun Y, Kim CD, Kim K. Toxicol Appl Pharmacol. 2014; 274:462–470.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download