Abstract
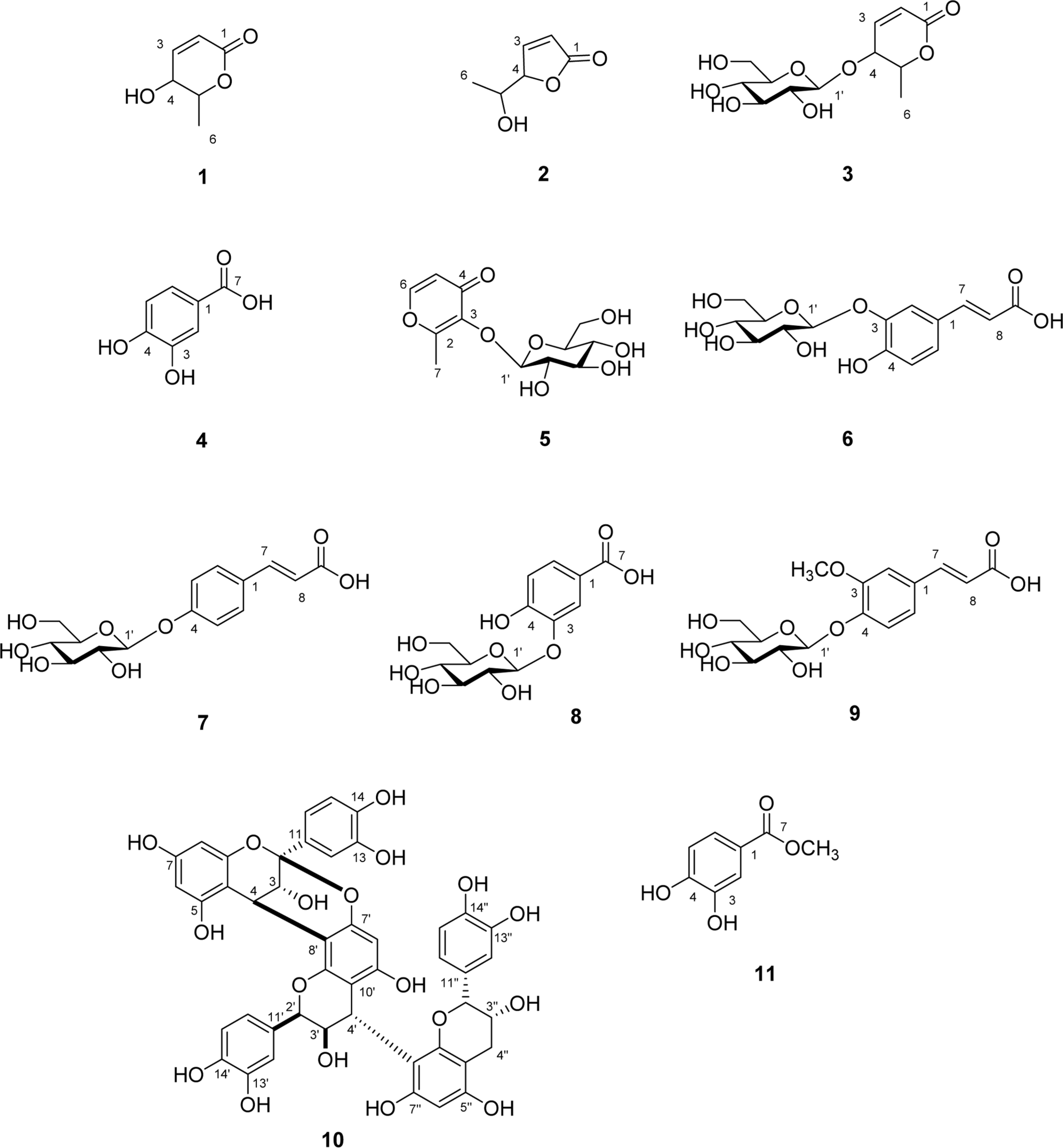
Eleven compounds (1–11) were isolated from the rhizomes of Osmunda japonica, and their structures were elucidated based on1H,13C-NMR and LC-IT-TOF MS data. Of these compounds, all compounds (1 – 11) have been previously reported, although five (6 – 9, 11) have not previously been isolated from this plant. The antioxidant activities of isolated compounds (1 – 11) were measured by DPPH and ABTS assays, and compound 10 showed the high antioxidant activity.
Go to : 
References
(1). Lee Y. N.New flora of Korea; Kyo-Hak Pub. Co: Seoul,. 2010. , p. 37.
(2). Kim M. S.., Woo K. W.., Lee K. H.., Lee H. J.., Lee S. Y.., Kang B. M.., Jeon B. H.., Cho J. H.., Cho H. W.Kor. J. Pharmacogn. 2016. 47:232–236.
(3). Li F.., Hu Y.Asian. J. Chem. 2012. 24:4964–4966.
(4). Zhu X. X.., Li Y. J.., Yang L.., Zhang D.., Chen Y.., Kmonickova E.., Weng X. G.., Yang Q.., Zidek Z.Chin. J. Integr. Med. 2013. 19:761–770.
(5). Shin S. L.., Lee C. H.Korean. J. Plant Res. 2010. 23:436–444.
(6). Kim M. S.., Lee Y. S.., Khoa D. B.., Kim H. Y.., Choi H. J.., Lim S. H.., Heo S. J.., Kwon S. B.., Park D. S.., Han S. S.., Kim S. M.Korean J. Pestic. Sci. 2004. 8:220–230.
(7). Jeong J. A.., Kwon S. H.., Lee C. H.Korean. J. Plant Res. 2007. 20:185–192.
(8). Heo C.., Chung J. H.., Jo B. K.., Kim H. P.., Heo M. Y. J.Appl. Pharmacol. 2003. 11:196–199.
(9). Numata A.., Hokimoto K.., Takemura T.., Katsuno T.., Yamamoto K.Chem. Pharm. Bull. 1984. 32:2815–2820.
(10). Numata A.., Takahashi C.., Fujiki R.., Kitano E.., Kitajima A.., Takemura T.Chem. Pharm. Bull. 1990. 38:2862–2865.
(11). Lee S. Y.., Kim K. H.., Lee I. K.., Lee K. H.., Choi S. U.., Lee K. R.Arch. Pharm. Res. 2012. 35:415–421.
(12). Zhou X. J.., Yan L. L.., Yin P. P.., Shi L. L.., Zhang J. H.., Liu Y. J.., Ma C.Food Chem. 2014. 164:150–157.
(13). Kwon J. H.., Kwon Y. M.., Choi S. E.., Park K. H.., Lee M. W.Nat. Prod. Sci. 2010. 16:10–14.
(14). Yamanaka M.., Shimomura K.., Sasaki K.., Yoshihira K.., Ishimaru K.Phytochemistry. 1995. 40:1149–1150.
(15). Jun H. I.., Jang H.., Ahn D.., Kim D. K.., Yang J. H.., Yun B. S.., Kim Y. S.Food Sci. Biotechnol. 2015. 24:2031–2034.
(16). Idowu T. O.., Ogundaini A. O.., Salau A. O.., Obuotor E. M.., Bezabih M.., Abegaz B. M.Phytochemistry. 2010. 71:2092–2098.
(17). Lee S. S.., Kim T. H.., Lee E. M.., Lee M. H.., Lee H. Y.., Chung B. Y.Food Chem. 2014. 156:312–318.
(18). Tiwari A. K.Current Science. 2001. 81:1179–1187.
(19). Wu J. Q.., Kosten T. R.., Zhang X. Y.Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013. 46:200–206.
(20). Pellegrini N.., Serafini M.., Colombi B.., Del Rio D.., Salvatore S.., Bianchi M.., Brighenti F. J.Nutr. 2003. 133:2812–2819.
(21). López J. J.., Jardin I.., Salido G. M.., Rosado J. A.Life Sci. 2008. 82:977–982.
(22). Gonzalez A.., Santofimia-Castaño P.., Rivera-Barreno R.., Salido G. M. J.Physiol Biochem. 2012. 68:181–191.
Go to : 
Table 1.
The antioxidant activities of O. japonica compounds (1 – 11)∗




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download