Abstract
In our program to search for new AMP-activated protein kinase (AMPK) activators from plants that exert potential anticancer property, we found that an EtOAc extract of Myristica fragrans (nutmeg) activated AMPK enzyme in human breast cancer MCF-7 cells. Two major diarylbutane-type lignans, macelignan and meso-dihydroguaiaretic acid (MDGA), were isolated as active principles from this extract. Treatment of breast cancer cells with two compounds induced cellular apoptosis, evidenced by cleavage of poly-(ADP-ribose) polymerase (PARP) and Ser 15 phosphorylation of p53. Moreover, macelignan and MDGA significantly inhibited the colony formation of MCF-7 breast cancer cells on soft agar. Intraperitoneal injection of macelignan and MDGA (20 mg/kg) suppressed the tumor growth of 4T1 mammary cancer cells. These results indicate that the chemopreventive effects of two major diarylbutane-type lignans from Myristica fragrans (nutmeg) may be associated with induction of apoptosis presumably through AMPK activation.
Breast cancer is the most common malignant tumor affecting women with more than one million new cases each year.12 Up to now, treatment of breast cancer remains a challenge. Available therapies including radiation, endocrine, and conventional chemotherapy are often limited by high toxicity, lower efficacy, therapeutic resistance, and therapy-related morbidity.3 Therefore, searching for more effective therapeutic agents with novel mechanisms of action is desired to combat breast cancer. Recent interest has focused on cell signaling pathways controlling both cell metabolism and cell growth.45 And also, the association of reduced cancer risk and edible food has also captured the interests of scientists.6
Adenosine monophosphate-activated protein kinase (AMPK), an energy sensing/signalling intracellular protein found in all eukaryotes, is a heterotrimeric serine/threonine protein kinase that is composed of a catalytic α-subunit and regulatory β-and γ-subunits.7 AMPK has been implicated in carcinogenesis because of the finding that a well-known tumor suppressor LKB1 (liver kinase B1) is an upstream activating kinase for AMPK, and many effects of LKB1 on tumor suppressing are likely to be mediated by AMPK.8 Moreover, it has been reported that AMPK activation could have therapeutic potential in breast cancer. Once activated, AMPK inhibits fatty acid synthesis and the AKT-mTOR pathway, and activates the p53-p21 axis. All these molecular mechanisms are thought to play a key role in breast carcinogenesis. Histological evaluation of AMPK signaling in primary breast cancer also showed the reduced signal of AMPK, compared with the strong expression in normal breast epithelium, in axillary node metastasis of two cohorts of patients.9
Myristica fragrans Houtt. (Myristicaceae), an aromatic evergreen tree cultivated in the India, South Africa, and other tropical countries, is widely used as a spice in many African and Asian countries.10 Lignans, which are known to exert many chemopreventive effects,11 were reported as the main chemical constituents of this plant. In this regard and as part of our program to search for new AMPK activators with chemopreventive activity from plants,12 we found that diarylbutane-type lignans from M. fragrans exhibited potential AMPK activation effects and strong growth inhibition activities against MCF-7 breast cancer cells. As major active principles, two diarylbutane-type lignans, macelignan (1)13 and MDGA (2)14, were isolated using bioassay-guided fractionation method. Herein we will report the isolation and structure elucidation of the two compounds as well as their anti-cancer effects and possible mechanisms of action.
Optical rotations were determined on a JASCO P-2000 polarimeter using a 100 mm glass microcell. NMR spectra were obtained on a Varian Unity Inova 600 MHz spectrometer with TMS as the internal standard at the Korea Basic Science Institute (KBSI, Gwangju Center, Korea). MS data were recorded on a Micromass QTOF2 (Micromass, Wythenshawe, UK) mass spectrometer. Silica gel (Merck, 63 - 200 µm particle size) and RP-18 (Merck, 40 - 63 µm particle size) were used for column chromatography (CC). TLC was carried out with silica gel 60 F254 and RP-18 F254 plates. All solvents used for extraction and isolation were of analytical grade.
The dried seeds of M. fragrans (nutmeg) were purchased at a folk medicine market in Gwangju city, Republic of Korea. The sample was identified by Prof. WK Oh at Seoul National University, and its specimen (No. 2012-0009) has been deposited at the Department of Pharmacy, Seoul National University, Republic of Korea.
The dried seeds of M. fragrans (5 kg) were extracted with EtOAc (10 L × 3 times) at room temperature. After concentration under reduced vacuum to give a darkness (550 g), this EtOAc-extract was directly subjected to a silica gel open column (20 × 30 cm) using a stepwise gradient of n-hexane/EtOAc (from 50:1, 40:1, 30:1, 20:1, 15:1, 10:1 to 0:1, 5 L for each system) to afford ten fractions (E.1-E.10) according to their TLC profiles. Compound 1 (macelignan, 15 g) was crystallized using a n-hexane: acetone system (1/1) from fraction 3 (E.3). Fraction 5 (E.5) was subjected to a RP-C18 open column (5.0 × 60 cm) using an isocratic of MeOH/H2O (v/v, 2:1), and resulted in the isolation of compound 2 (MDGA, 5 g).
White crystal (n-hexane/acetone, 1/1); 1H-NMR (600 MHz, CDCl3) δH: 6.82 (1H, d, J = 7.9 Hz, H-5′), 6.72 (1H, d, J = 7.8 Hz, H-5), 6.65 (lH, d, J = 1.8 Hz, H-2′), 6.64 (lH, dd, J = 1.8, 7.9 Hz, H-6′), 6.61 (lH, d, J = 1.6 Hz, H-2), 6.60 (lH, dd, J = 1.6, 7.8 Hz, H-6), 5.91 (1H, d, J = 2.1 Hz, O-CH2-O), 5.48 (lH, s, 4-OH), 3.86 (3H, s, OCH3), 2.72 (2H, dd, J = 4.9, 13.7 Hz, H-7a/7′a), 2.28 (1H, dd, J = 9.2, 13.7 Hz, H-7b/7′b), 2.25 (lH, dd, J = 9.4, 13.7 Hz), 1.67-1.77 (2H, m, H-8/8′), 0.83 (3H, d, J = 6.6 Hz, H-9′), 0.82 (3H, d, J = 6.6 Hz, H-9); 13C NMR (150 MHz, CDCl3): 133.8 (C-l), 135.7 (C-l′), 111.7 (C-2), 108.1 (C-2′), 146.6 (C-3), 147.7 (C-3′), 143.8 (C-4), 145.6 (C-4′), 114.2 (C-5), 109.4 (C-5′), 121.8 (C-6/6′), 39.2/39.0 (C-7/7′), 39.5/39.4 (C-8/8′), 16.3/16.2 (C-9/9′), 100.6 (OCH2-O), and 56.0 (-OCH3).
White powder; 1H-NMR (600 MHz, CDCl3) δH: 6.82 (2H, d, J = 8.2 Hz, H-5/5′), 6.65 (2H, dd, J = 1.5, 8.4 Hz, H-6/6′), 6.61 (2H, d, J = 1.5 Hz, H-2/2′), 3.85 (6H, s, OCH3), 2.73 (2H, dd, J = 5.1, 13.6 Hz, H-7b/7′b), 2.28 (2H, dd, J = 9.3, 13.6 Hz, H-7a/7′a), 1.75 (2H, m, H-8/8′), 0.84 (6H, d, J = 6.6 Hz, H-9/9′); 13C-NMR (100 MHz, CDCl3) δC: 133.8 (C-1/1′), 111.4 (C-2/2′), 146.3 (C-3/3′), 143.5 (C-4/4′), 113.9 (C-5/5′), 121.7 (C-6/6′), 39.0 (C-7/7′), 39.2 (C-8/8′), 16.3 (C-9/9′), 56.9 (3-/3-OMe).
McCoy's 5A medium, Dulbecco's Modified Eagle medium (DMEM), L-glutamine, gentamicin, and fetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad, CA, USA). Polyvinylidene difluoride (PVDF) membrane was obtained from Millipore (Bedford, MA, USA). 3-[4,5-Dimethylthiazol-2-thiazoyl]-2,5-diphenyltetrazolium bromide (MTT) was bought from Sigma-Aldrich (St. Lous, MO, USA). Antibodies against phospho-p53 (ser-15), phospho-AMPK, cleaved PARP, and PARP were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA), and goat anti-mouse IgG HRP, goat anti-rabbit IgG HRP and bovine anti-goat IgG HRP were from Santa Crutz Biotechnology (Santa Cruz, CA, USA). Fixation/permeabilization solution (Cytofix/Cytoperm) was purchased from BD biosciences (San Jose, CA, USA).
The screening cell lines (MCF-7 and MDA-MB-231 human breast carcinoma cells, and the multidrug-resistant cell lines (TAMR/MCF-7 and ADR/MCF-7) were maintained at 37℃ in a humidified atmosphere containing 5% CO2. To establish the TAMR-MCF7 cells, a stepwise drug selection was continued until the MCF7 cell population could sustain viability and proliferation when challenged with 3 mM of tamoxifen. The established TAMR-MCF7 cells were maintained in DMEM with 10% charcoal/dextran-treated FBS and 3 mM of tamoxifen. All the media used were McCoy's 5A and DMEM supplemented with 10% heat-inactivated fetal bovine serum, 4.5 g/L D-glucose, 100 mg/L sodium pyruvate and L-glutamine. The cells were subcultured every 3 days using the standard trypsinization procedure.
The cell viability was assessed using a 4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate (WST-1) based cytotoxicity assay kit to determine the IC50 of the isolated compounds (Daeil lab service Co., Ltd, Korea). In these assays, 1 × 104 (MCF-7, TAMR/MCF-7, MDA-MB-231) or 1.5 × 104 (ADR/MCF-7) cells in 100 µL of the culture medium per well were seeded in 96-well plates and allowed to adhere for 24 hr prior to treatment. The final concentration of DMSO in the culture medium was maintained at 0.05% (v/v) to avoid solvent toxicity. Subsequently, 10 µL of the kit solution was added to each well of the plate and the absorbance was measured at 450 nm using an ELISA reader. The survival percentages are defined as the absorbance in the experiment wells compared to that in the control wells. The cytotoxicity results are expressed as the mean standard deviation and represent the concentration inhibiting 50% cell growth (IC50). Each experiment was carried out three times in triplicate.
MCF-7 cells (2 × 104) were exposed to different dose of macelignan or MDGA in 1 mL of 0.3% basal medium Eagles (BME) agar containing 10% FBS, 2 mM L-glutamine, and 25 ug/mL gentamicin. Colonies were scored after 2 weeks of incubation with or without treatment of the lignans at 37℃ in 5% CO2 in air. Each experiment was performed in triplicate.
MCF-7 cells were incubated with appropriate concentrations of compounds for indicated time and then lysed in EBC lysis buffer [50 mM Tris-HCl (pH 7.6), 120 mM NaCl, 1 mM EDTA (pH 8.0), 0.5% NP-40, and 50 mM sodium fluoride]. Cell debris was removed by centrifugation at 12,000 rpm for 15 min, at 4℃. Protein concentrations in the cell lysates were determined using a Bio-rad protein assay kit. The proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride membrane (PVDF). The membranes were blocked and hybridized with the appropriate primary antibody overnight at 4℃. The immunoreactive antigen was then recognized by using a horseradish peroxidase-labeled anti-rabbit IgG and an enhanced chemiluminescence detection kit.
Six-week-old female Balb/c mice (Joongang Experimental Animal Co., Seoul, Korea) were anesthesized with 50 mg/kg pentobarbital and the rudimentary mammary ducts was cleared. 4T1 mammary cancer cells were harvested by trypsinization and centrifuged and resuspended in DMEM at a density of 3 × 106/100 µl. 100 µl cell mixtures were injected into the cleared fat pad. Compounds treatment began from 1 week after 4T1 cell transplantation. Macelignan and MDGA (20 mg/kg) or an equal volume of the vehicle was intraperitoneally injected every two days for a total of 8 times. At the end of the treatment period, animals were sacrificed and solid tumors were excised for further studies. Body weights were recorded daily. All experiments were conducted under protocols approved by the Animal Care and Use Committee at College of Dentistry of Chosun University.
During our searching novel AMPK activators from plants, we found that an EtOAc extract of M. fragrans exhibited a potent effect on AMPK activation. Bioassay-guided fractionation using repeated silica gel and RP-C18 column chromatoghraphy resulted in a wide range of lignans that strongly increased phosphorylation level of the Thr172 residue of AMPK α subunit in MCF-7 breast cancer cells. These compounds were also screened for cytotoxicity against MCF-7 cells. The results suggested that the diarylbutane-type lignans showed the most potent inhibition effect on cell growth (> 90% at the concentration of 20 µg/mL). By analysis of optical rotation values, 1D- and 2D-NMR spectroscopic and mass spectrometry data as well as comparing these data with literature values, two major b ioactive compounds were identified as macelignan and MDGA.1314 Furthermore, HPLC analysis (Fig. 1) showed the high content of macelignan and MDGA at EtOAc layer of Myristica fragrans. Thus, the two compounds were isolated excessively for further studies.
We utilized MCF-7 cell lines to investigate the effects of macelignan and MDGA on AMPK activation, and AICAR was used as positive control. As shown in Fig. 2, western blot analysis of a time course assay with MCF-7 cells demonstrated that macelignan and MDGA increased the phosphorylation of the Thr172 residue of the AMPK α subunit in a time-dependent manner at 5 µg/mL. When the dose-dependent effects of macelignan and MDGA on AMPK activation were checked, the phosphorylation of AMPK at MCF-7 cancer cells was increased with dose-dependently at the concentrations of 1, 2.5, 5, and 10 µg/mL. These data indicated that both compounds can stimulate the phosphorylation and activity of AMPK at dose-dependent and time-dependent manners.
In order to evaluate the effects of macelignan and MDGA on the growth of human breast cancer cells, MCF-7 (estrogen receptor-positive), MDA-MB-231 (estrogen receptor-negative), tamoxifen-resistant MCF-7 (TAMR/MCF-7), and adriamycin-resistant MCF-7 (MCF-7/ADR) cancer cells were treated with various concentrations of two compounds. As shown in Table 1, both compounds showed significant growth inhibitory effects which are stronger than that of positive control in all tested cell lines at a dose-dependent manner. Macelignan and MDGA showed similar inhibition patterns on different breast cancer lines with IC50 values ranging from 5.97 ± 0.33 to 7.67 ± 0.27 µg/mL.
Cell colony formation has been found to be a more sensitive parameter of toxicity than cell viability because colony formation is assessed while the cells are in a state of proliferation, and thus more susceptible to toxic effects.15 Soft agar colony formation assays have been used to determine the growth and drug sensitivity of MCF-7 cells.1617 In this study, MCF-7 cells were divided into control group and sample group treated with different concentrations of two lignans. The cells were cultured for two weeks in the presence of two lignans. As shown in Fig. 3, both macelignan and MDGA significantly inhibited the colony-formation ability of MCF-7 cells in a dose-dependent manner. The colony formation of MCF-7 cells after treatment of macelignan and MDGA was significantly inhibited compared with the control group. The inhibition was evident not only in colony number but also in colony size. Colony-formation ability of macelignan and MDGA at the final concentration of 20 µg/mL was approximately 0%, i.e. almost no colony was observed. Significantly (P<0.05) reduced colony-formation ability was observed for lignan groups at the concentrations of 5 and 10 µg/mL.
As MCF-7 cancer cells do not express functional caspase 3, it appeared possible that the absence of this executioner caspase might prevent the morphological changes and DNA fragmentation associated with apoptotic cell death. To confirm whether macelignan and MDGA induce apoptosis in MCF-7 cells or not, we detected PARP cleavage by western blot analysis. Anti-PARP antibody recognized an approximately 85-kDa band, which may have corresponded to the 85-kDa fragmentation of PARP (cleaved PARP) in the cells. This 85-kDa band was not seen in the untreated cells and slightly appeared at 1 h after 5 µg/mL treatment of each lignan, then was increased. gradually until 12 h after treatment. Fig. 4A., indicated that PARP cleavage was clearly shown in MCF-7 cells exposed to macelignan and MDGA as well as AICAR which was used as positive control.
The ability of p53 to induce apoptosis in response to abnormal proliferative signals and stress is the one of critical importance for its tumor suppression function. The phosphorylated site of p53 at ser 15 plays an important role for the p53 activation and stabilization.18 Hence, we tested effects of the two lignans on Ser 15-phosphorylation of p53. Macelignan at the concentration of 10 µg/mL increased phosphorylated p53, but MDGA did not affect it (Fig. 4B). These data demonstrate that macelignan, but not MDGA, induces apoptosis presumably through p53-dependent pathway.
To investigate whether macelignan and MDGA can inhibit the tumor in vivo, we tested inhibitory effects of two lignans on 4T1 mammary cancer cells in six-week-old female Balb/c mice. As shown in Fig. 5A and 5B, differences in tumor growth were significant between the macelignan and MDGA injection group and the control group from 8 days after injection. On the 8th day, the weights and volumes of the tumors in the macelignan and MDGA group were significantly less than those in the control group (P<0.05). Furthermore, the H & E stain showed that macelignan and MDGA induced the apoptosis of cancer cells (Fig. 5C).
As a member of the metabolite-sensing protein kinase family, AMPK plays a key role in cell survivals and apoptosis.19 AMPK demonstrates the potential as a novel therapeutic target for the treatment of cancers,2021 which was also supported by the presence of the tumor-suppressors such as LKB1 in upstream and p53 and TSC2 (sclerosis complex subunit 2) in downstream.
The species M. fragrans has been used traditionally for spices and various medicinal purposes with carminative, hypolipidaemic, antithrombotic, anxiogenic, antiplatelet aggregating, antifungal, anti-ulcerogenic, antitumor, and anti-inflammatory activities.22 Phytochemical investigations showed that lignans are the main secondary metabolites of this plant with a wide spectrum of biological activities including anti-oxidant, anti-inflammatory, anti-carcinogenic, anti-diabetic, and hepatoprotective effects.23 During our effort to discover new AMPK activators from nature, MeOH extract of M. fragrans significantly increased phosphorylation level of the Thr172 residue of AMPK α subunit in MCF-7 breast cancer cells. Successive chromatographic procedures and extensive spectroscopic analysis were applied to identify the major anti-cancer principles of M. fragrans. Both macelignan and MDGA could effectively induced apoptosis, evidenced by cleavage of poly-(ADP-ribose) polymerase (PARP) and Ser 15 phosphorylation of p53, and both compounds also significantly inhibited the colony-formation ability of MCF-7 cells. In addition, intraperitoneal injection of macelignan and MDGA (20 mg/kg) suppressed the tumor growth of 4T1 mammary cancer cells. These results indicate that the underlying molecular mechanism of the anticancer effects of macelignan and MDGA may be associated with AMPK activation. This is the first report on the anticancer property in vivo and the possible mechanism of macelignan and MDGA against MCF-7 cell lines. It also indicates the potential of macelignan and MDGA as AMPK activators for the prevention and treatment of breast cancer.
Figures and Tables
Fig. 1
A representative HPLC profile of major compounds from the total EtOAc layer of Myristica fragrans with detections at 205 and 280 nm. Key to peak identity: (1) macelignan, (2) MDGA. Chromatographic method used: 0 - 40 min (50 - 70% MeOH), 40 - 52 min (70 - 100% MeOH), 52 - 60 min (100% MeOH).

Fig. 2
Phosphorylation of AMPK by macelignan and MDGA in MCF-7 cells. Cells were seeded using DMEM supplemented with 10% heat-inactivated fetal bovine serum without antibiotic and cultured for 24 hrs at 37℃ in humidified air condition containing 5% CO2. The cells were then starved with serum free DMEM media for 24 hrs and treated with 5 µg/ml of macelignan and MDGA for 10 min, 30 min, 1 hr, and 2 hrs (the left panels of A and B) or treated with 0, 1, 2.5, 5, and 10 µg/ml of macelignan and MDGA (the right panels of A and B), respectively. The cells were harvested with cold PBS and lysed with 1 × NP40 lysis buffer. Proteins in whole cell lysates were separated by SDS-PAGE and immunoblotted with antibodies against phospho-AMPK and total AMPK. AICAR, an AMPK activator, was used as a positive control in this experiment. Data are representative of three independent experiments that gave similar results.

Fig. 3
Inhibitory effects of macelignan and MDGA on colony formation of MCF-7cells. MCF-7 cells were subjected to soft agar assays in the presence of macelignan and MDGA. Cells (8 × 103/mL) were exposed to 1, 5, 10, and 20 µg/ml of macelignan and MDGA, respectively, in 1 ml of 0.3% basal medium Eagle (BME) agar containing 10% FBS, 2 mM L-glutamine, and 25 µg/ml gentamicin. The cultures were maintained at 37℃, in a 5% CO2 incubator for 10-14 days, and the cell colonies were scored using a microscope and the Image-Pro PLUS computer software program (Media Cybernetics, Silver Spring, MD).
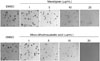
Fig. 4
Effects of macelignan and MDGA on PARP cleavage and p53 phosphorylation on MCF-7 cells. Cells were cultured using DMEM supplemented with 10% heat-inactivated fetal bovine serum for 24 hrs at 37℃. The cells were then starved with serum free DMEM media for 24 hrs and treated with 5 µg/ml of macelignan and MDGA for 1, 3, 6, and 12 hrs (the upper panels of A and B) for cleaved PARP and treated with 0, 1, 2.5, 5, and 10 µg/ml of macelignan and MDGA for 30 min for phospho-P53 (the lower panels of A and B), respectively. AICAR was used as a positive control. Data are representative of three independent experiments that gave similar results.
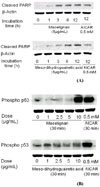
Fig. 5
In vivo anti-cancer effect on 4T1 mammary cancer cells. Six-week-old female Balb/c mice were anesthesized with 50 mg/kg pentobarbital and the rudimentary mammary ducts was cleared. 4T1 mammary cancer cells were harvested by trypsinization and centrifuged and resuspended in DMEM at a density of 3 × 106/100 µl. 100 µl cell mixtures were injected into the cleared fat pad. Macelignan and MDGA (20 mg/kg) or an equal volume of the vehicle (n = 5 - 6 mice each group) were intraperitoneally injected by every two days for a total of 8 times. At the end of the treatment period, animals were sacrificed and solid tumors were excised for further studies. Body weights were recorded daily.
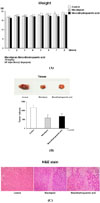
Table 1
Anti-proliferative effects of diarylbutane lignans from M. fragrans in MCF-7, MDA-MB-231, TAMR/MCF-7, and ADR/MCF-7

Acknowledgements
This work was supported in part by grants from the Marine Biotechnology Program of the Ministry of Oceans and Fisheries (PJT200669) and the Korea Bioactive Natural Material Bank (NRF-2012M3A9B8021570) of the National Research Foundation of Korea (NRF), which is funded by the Korean government.
References
1. Evans DGR, Howell A. Breast Cancer Res. 2007; 9:213–220.
2. Zhou W, Guan X, Wang L, Liao Y, Huang J. J Cancer Res Clin Oncol. 2012; 138:2085–2093.
3. Nagalingam A, Arbiser JL, Bonner MY, Saxena NK, Sharma D. Breast Cancer Res. 2012; 14:R35.
4. Guppy A, Jamal-Hanjani M, Pickering L. Future Oncol. 2011; 7:727–736.
5. Li BX, Yamanaka K, Xiao X. Bioorg Med Chem. 2012; 20:6811–6820.
6. Fortes C, Forastiere F, Farchi S, Mallone S, Trequattrinni T, Anatra F, Schmid G, Perucci CA. Nutr Cancer. 2003; 46:30–37.
7. Tran TP, Kim HG, Choi JH, Na MK, Jeong HG. Phytomedicine. 2013; 20:622–631.
8. Nguyen HB, Babcock JT, Wells CD, Quilliam LA. Oncogene. 2013; 32:4100–4109.
9. Hadad SM, Baker L, Quinlan PR, Robertson KE, Bray SE, Thomson G, Kellock D, Jordan LB, Purdie CA, Hardie DG, Fleming S, Thompson AM. BMC Cancer. 2009; 9:307–315.
10. Lee KE, Mun S, Pyun HB, Kim MS, Hwang JK. Biol Pharm Bull. 2012; 35:1669–1675.
11. Pan JY, Chen SL, Yang MH, Wu J, Sinkkonen J, Zou K. Nat Prod Rep. 2009; 26:1251–1292.
12. Nguyen PH, Le TVT, Kang HW, Chae J, Kim SK, Kwon KI, Seo DB, Lee SJ, Oh WK. Bioorg Med Chem Lett. 2010; 20:4128–4131.
13. Woo WS, Shin KH, Wagner H, Lotter H. Phytochemistry. 1987; 26:1542–1543.
14. Nakatani N, Ikeda K, Kikuzaki H, Kido M, Yamaguchi Y. Phytochemistry. 1988; 27:3127–3129.
15. Hashimura T, Yoshida O. Jpn J Cancer Res. 1985; 76:321–323.
16. Li Q, Ling Y, Yu L. J Cancer Res Clin Oncol. 2012; 138:1073–1079.
17. Zhang X, Zhang S, Liu Y, Liu J, Ma Y, Zhu Y, Zhang J. Eur J Cancer. 2012; 48:1581–1592.
18. Dumaz N, Meek DW. EMBO J. 1999; 18:7002–7010.
19. Hardie DG. Curr Opin Cell Biol. 2005; 17:167–173.
20. Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, Lee IK, Viollet B, Youn JH, Lee HK, Lee KU. Nat Med. 2004; 10:727–733.
21. Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. FASEB J. 2005; 19:786–788.
22. Lee DH, Lee TH, Jung CH, Kim YH. Cell Signal. 2012; 24:2216–2225.
23. Choi EJ, Kang YG, Kim J, Hwang JK. Biol Pharm Bull. 2011; 34:748–754.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download