Abstract
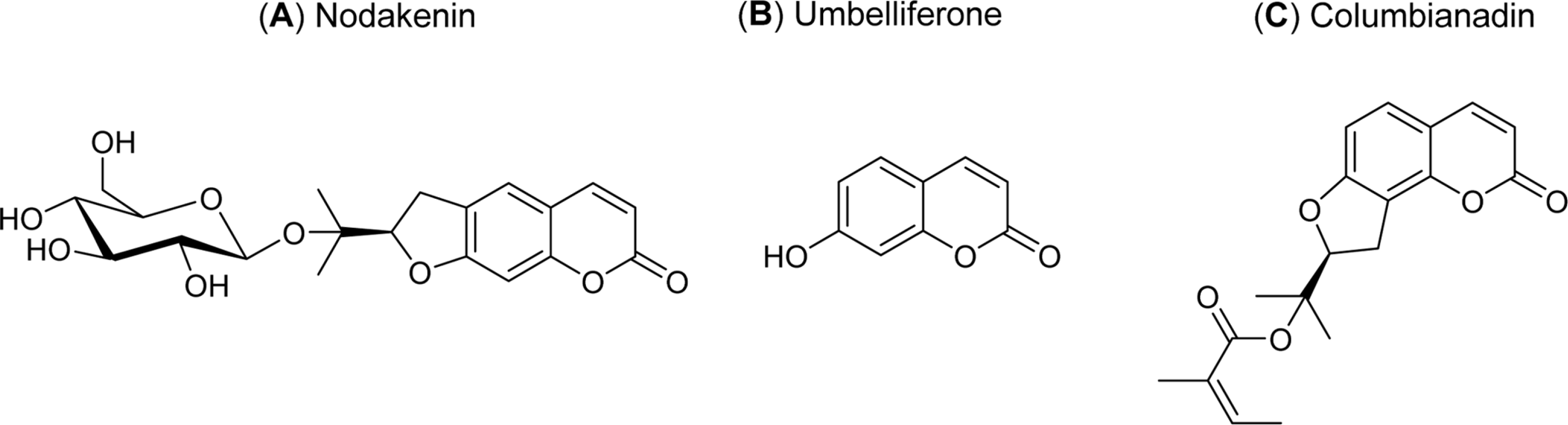
Angelica decursiva has been utilised as remedy for controlling the airway inflammatory diseases in folk medicine. We investigated whether nodakenin, columbianadin, and umbelliferone isolated from the roots of Angelica decursiva inhibit the gene expression and production of MUC5AC mucin from human airway epithelial cells. Confluent NCI-H292 cells were pretreated with nodakenin, columbianadin or umbelliferone for 30 min and then stimulated with epidermal growth factor (EGF), phorbol 12-myristate 13-acetate (PMA) or tumor necrosis factor-α (TNF-α) for 24 h. The MUC5AC mucin gene expression was measured by reverse transcription – polymerase chain reaction (RT-PCR). Production of MUC5AC mucin protein was measured by enzyme-linked immunosorbent assay (ELISA). The results were as follows: (1) Nodakenin did not affect the expression of MUC5AC mucin gene induced by EGF, PMA or TNF-α. Columbianadin inhibited the expression of MUC5AC mucin gene induced by EGF or PMA. However, umbelliferone inhibited the expression of MUC5AC mucin gene induced by EGF, PMA or TNF-α; (2) Nodakenin also did not affect the production of MUC5AC mucin protein induced by EGF, PMA or TNF-α. Columbianadin inhibited the production of MUC5AC mucin protein induced by PMA. However, umbelliferone inhibited the production of MUC5AC mucin protein induced by EGF, PMA or TNF-α. These results suggest that, among the three compounds investigated, umbelliferone only inhibits the gene expression and production of MUC5AC mucin stimulated by various inducers, by directly acting on airway epithelial cells, and the results might explain the traditional use of Angelica decursiva as remedy for diverse inflammatory pulmonary diseases.
Go to : 
References
(1). Voynow J. A.., Rubin B. K.Chest. 2009. 135:505–512.
(2). Lee H. J.., Ryu J.., Park S. H.., Seo E. K.., Han A. R.., Lee S. K.., Kim Y. S.., Hong J. H.., Seok J. H.., Lee C. J.Arch. Pharm. Res. 2015. 38:620–627.
(3). Seo H. S.., Sikder M. A.., Lee H.J.., Ryu J.., Lee C. J.Biomol. Ther (Seoul). 2014. 22:525–531.
(4). Sikder M. A.., Lee H. J.., Lee S. Y.., Bae H. S.., Kim J. H.., Chang G. T.., Lee C. J.Biomol. Ther (Seoul). 2011. 19:320–323.
(5). Lee H. J.., Lee S. Y.., Kim Y. S.., Jeon B. K.., Lee J. W.., Bae H. S.., Lee C. J.Biomol. Ther (Seoul). 2010. 18:396–401.
(6). Lee H. J.., Lee S. Y.., Jeon B. K.., Lee J. W.., Kim Y. S.., Lee M. N.., Lee C. J.Biomol. Ther (Seoul). 2010. 18:294–299.
(7). Jang I. M.Treatise on asian herbal medicines; Haksul-pyunsu-kwan in Research institute of natural products of Seoul National University: Seoul,. 2003. , p. 2759.
(8). Lim H. J.., Lee J. H.., Choi J. S.., Lee S. K.., Kim Y. S.., Kim H. P. J.Ethnopharmacol. 2014. 155:1353–1361.
(9). Park S. J.., Cha H. S.., Lee Y. H.., Kim W. J.., Kim D. H.., Kim E. C.., Lee K. H.., Kim T. J.Biosci. Biotechnol. Biochem. 2014. 78:1568–1571.
(10). Vasconcelos J. F.., Teixeira M. M.., Barbosa-Filho J. M.., Agra M. F.., Nunes X. P.., Giulietti A. M.., Ribeiro-Dos-Santos R.., Soares M. B.Eur. J. Pharmacol. 2009. 609:126–131.
(11). Xiong Y.., Wang J.., Yu H.., Zhang X.., Miao C.., Ma S.Immunopharmacol. Immunotoxicol. 2014. 36:341–348.
(12). Rogers D. F.., Barnes P. J.Ann. Med. 2006. 38:116–125.
(13). Li J. D.., Dohrman A. F.., Gallup M.., Miyata S.., Gum J. R.., Kim Y. S.., Nadel J. A.., Prince A.., Basbaum C. B.Proc. Natl. Acad. Sci. USA. 1997. 94:967–972.
(14). Takeyama K.., Dabbagh K.., Lee H. M.., Agusti C.., Lausier J. A.., Ueki I. F.., Grattan K. M.., Nadel J. A.Proc. Natl. Acad. Sci. USA. 1999. 96:3081–3086.
(15). Shao M. X.., Ueki I. F.., Nadel J. A.Proc. Natl. Acad. Sci. USA. 2003. 100:11618–11623.
(16). Song K. S.., Lee W. J.., Chung K. C.., Koo J. S.., Yang E. J.., Choi J. Y.., Yoon J. H. J.Biol. Chem. 2003. 278:23243–23250.
(17). Takeyama K.., Dabbagh K.., Shim J. J.., Dao-Pick T.., Ueki I. F.., Nadel J. A. J.Immunol. 2000. 164:1546–1552.
(18). Hong D. H.., Petrovics G.., Anderson W. B.., Forstner J.., Forstner G.Am. J. Physiol. 1999. 277:G1041–G1047.
(19). Hewson C. A.., Edbrooke M. R.., Johnston S. L. J.Mol. Biol. 2004. 344:683–695.
(20). Park S. J.., Kang S. Y.., Kim N. S.., Kim H. M.Immunopharmacol. Immunotoxicol. 2002. 24:211–226.
(21). Fischer B. M.., Rochelle L. G.., Voynow J. A.., Akley N. J.., Adler K. B.Am. J. Respir. Cell Mol. Biol. 1999. 20:413–422.
(22). Cohn L.., Whittaker L.., Niu N.., Homer R. J.Novartis Found. Symp. 2002. 248:201–213.
Go to : 
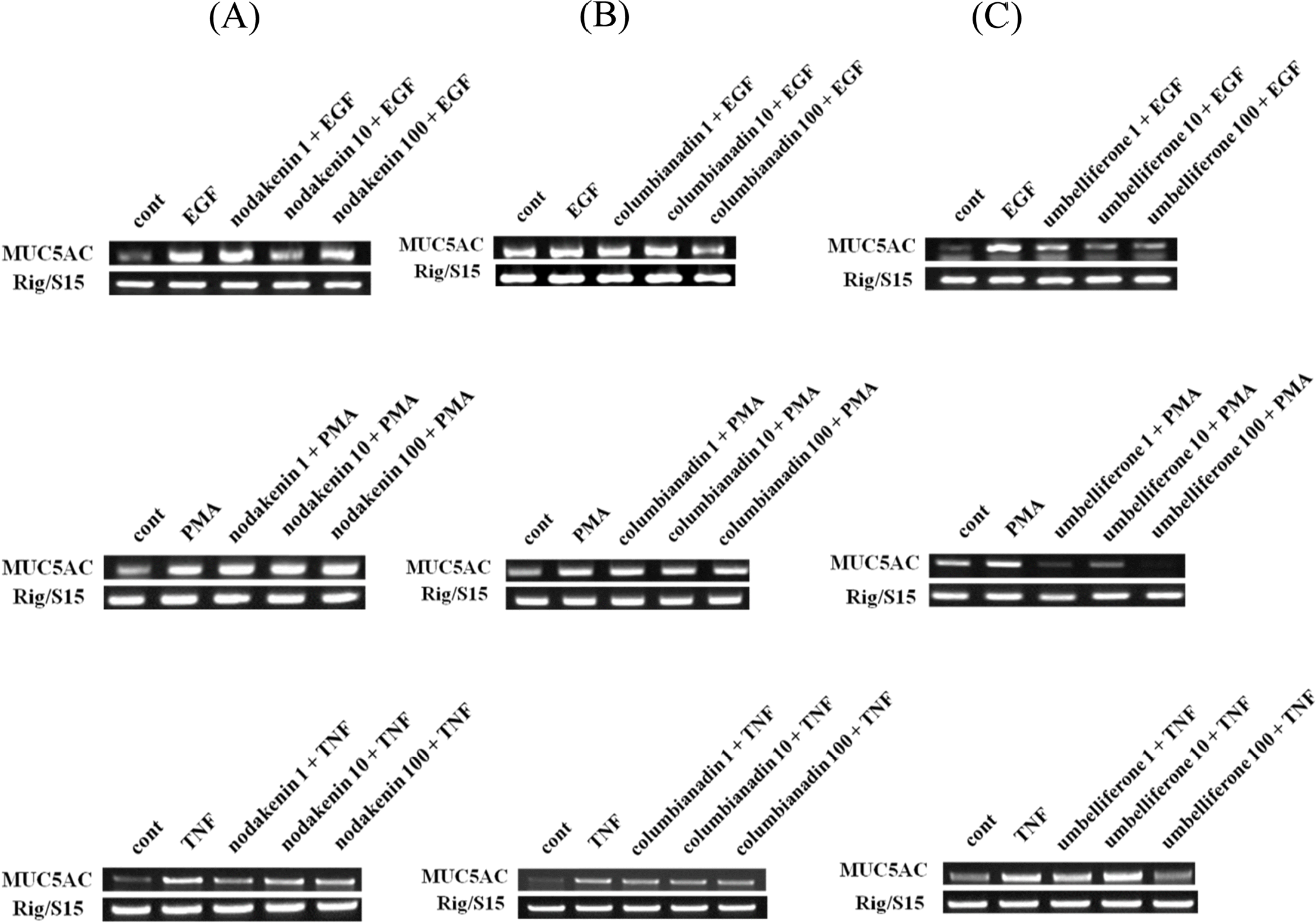 | Fig. 2.Effect of nodakenin, columbianadin or umbelliferone on EGF-, PMA- or TNF-α-induced MUC5AC gene expression in NCI-H292 cells. NCI-H292 cells were pretreated with varying concentrations (1, 10, and 100 µM) of nodakenin, columbianadin or umbelliferone for 30 min and then stimulated with EGF (25 ng/mL), PMA (10 ng/mL) or TNF-α (0.2 nM) for 24 h. MUC5AC gene expression was measured by RT-PCR. Three independent experiments were performed and the representative data were shown (cont: control, concentration unit is µM). |
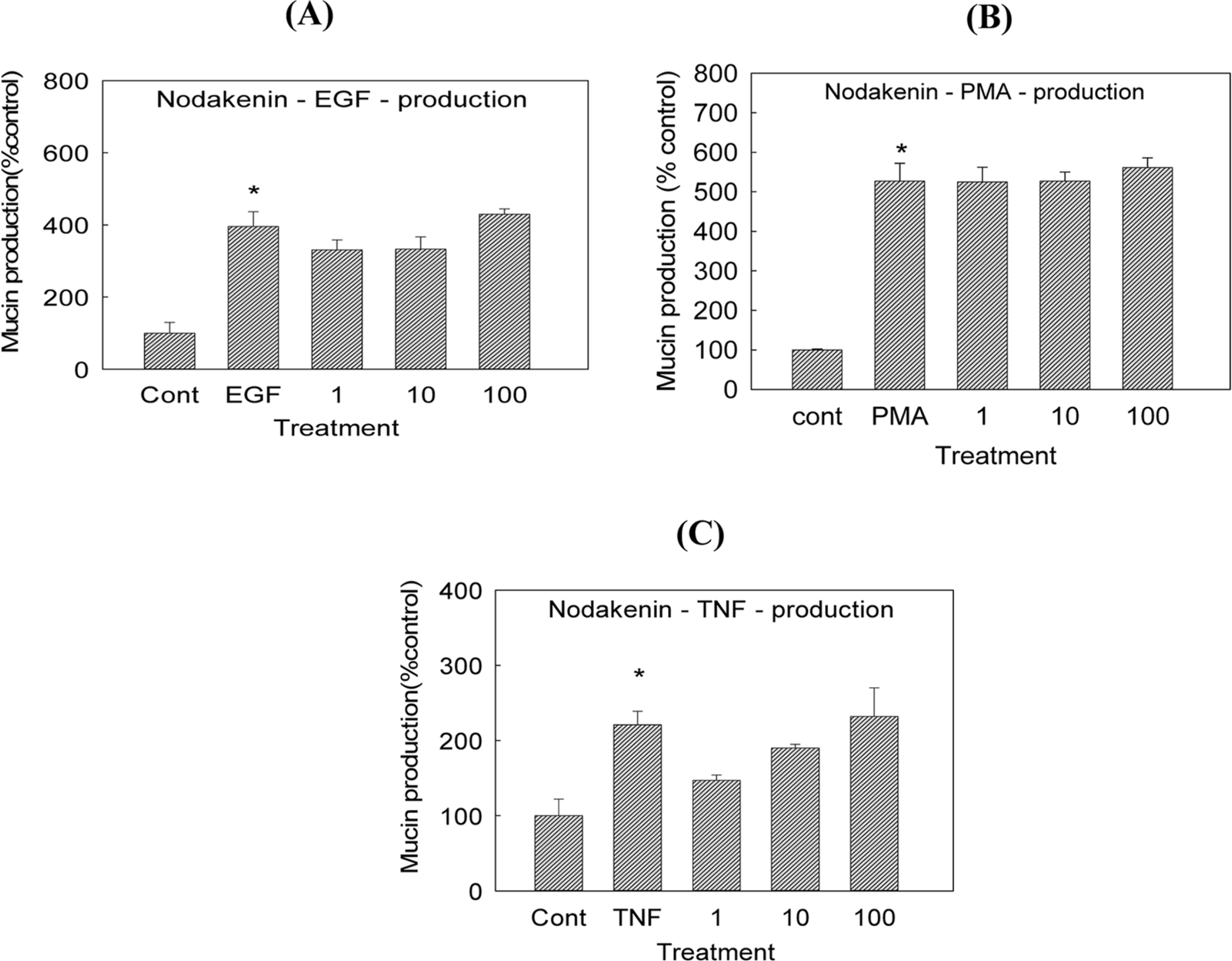 | Fig. 3.Effect of nodakenin on EGF-, PMA- or TNF-α-induced MUC5AC mucin production in NCI-H292 cells. NCI-H292 cells were pretreated with varying concentrations (1, 10, and 100 µM) of nodakenin for 30 min and then stimulated with EGF (25 ng/mL), PMA (10 ng/mL) or TNF-α (0.2 nM) for 24 h. Cell lysates were collected for measurement of MUC5AC mucin production by ELISA. Each bar represents a mean ± S.E.M. of 3 culture wells in comparison with that of control set at 100%. Three independent experiments were performed and the representative data were shown ∗ significantly different from control (p < 0.05) (cont: control, concentration unit is µM). |
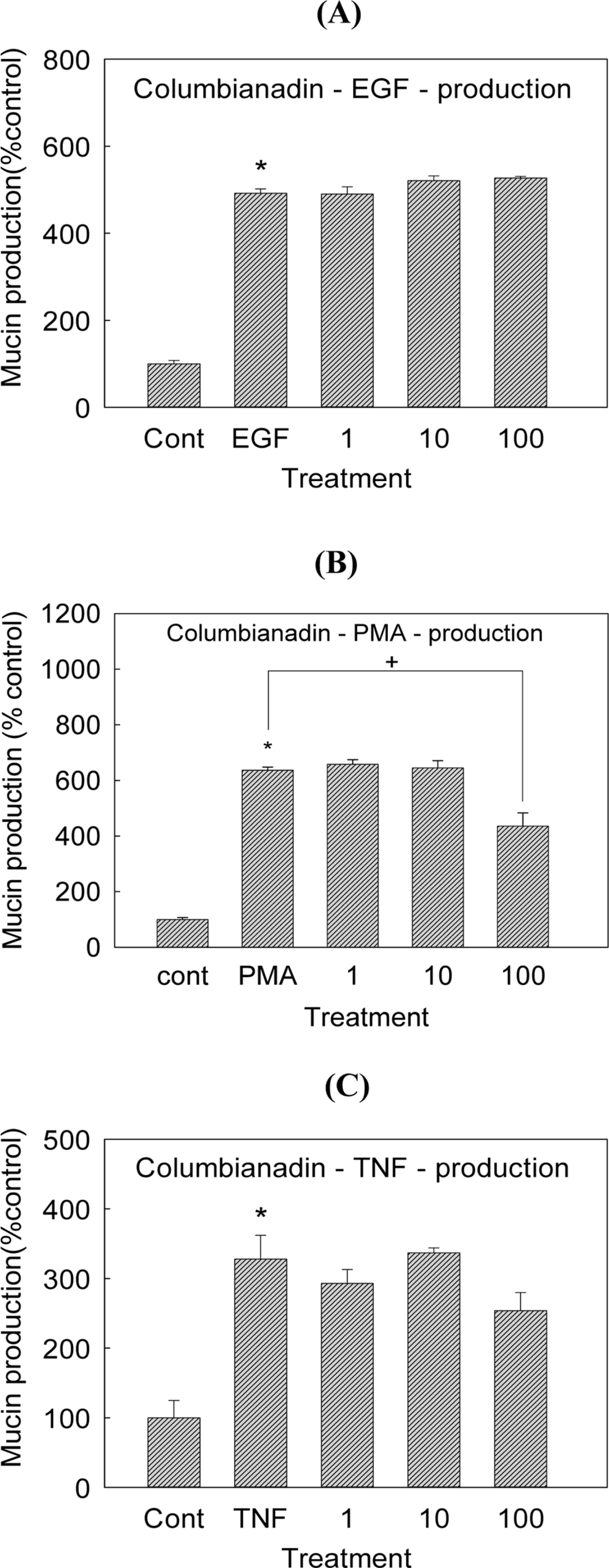 | Fig. 4.Effect of columbianadin on EGF-, PMA- or TNF-α-induced MUC5AC mucin production in NCI-H292 cells. NCI-H292 cells were pretreated with varying concentrations (1, 10, and 100 µM) of columbianadin for 30 min and then stimulated with EGF (25 ng/mL), PMA (10 ng/mL) or TNF-α (0.2 nM) for 24 h. Cell lysates were collected for measurement of MUC5AC mucin production by ELISA. Each bar represents a mean ± S.E.M. of 3 culture wells in comparison with that of control set at 100%. Three independent experiments were performed and the representative data were shown. ∗ significantly different from control (p < 0.05). + significantly different from EGF, PMA or TNF-α alone (p<0.05) (cont: control, concentration unit is µM). |
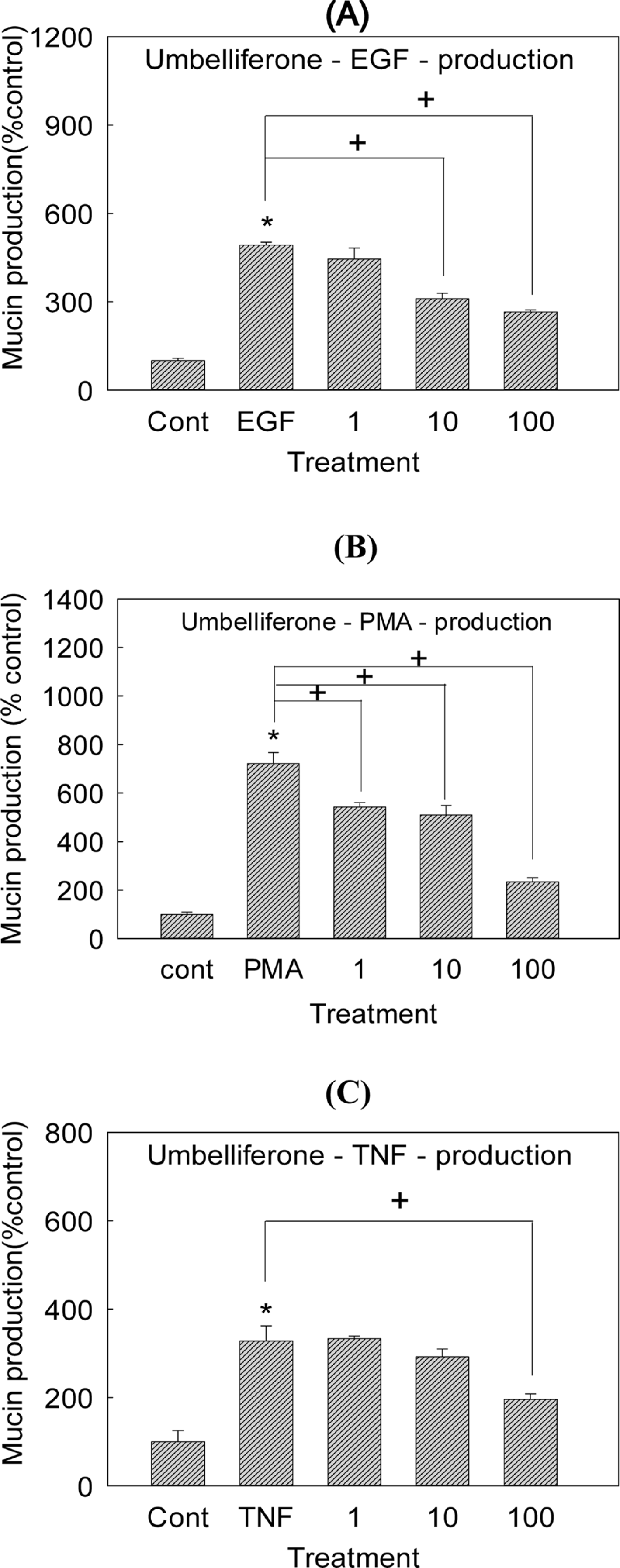 | Fig. 5.Effect of umbelliferone on EGF-, PMA- or TNF-α-induced MUC5AC mucin production in NCI-H292 cells. NCI-H292 cells were pretreated with varying concentrations (1, 10, and 100 µM) of umbelliferone for 30 min and then stimulated with EGF (25 ng/mL), PMA (10 ng/mL) or TNF-α (0.2 nM) for 24 h. Cell lysates were collected for measurement of MUC5AC mucin production by ELISA. Each bar represents a mean ± S.E.M. of 3 culture wells in comparison with that of control set at 100%. Three independent experiments were performed and the representative data were shown. ∗ significantly different from control (p < 0.05). + significantly different from EGF, PMA or TNF-α alone (p < 0.05) (cont: control, concentration unit is µM). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download