Abstract
The aim of this study was to investigate the effect and action mechanism of quercetin on penile corpus cavernosum smooth muscle (PCCSM). PCCSM precontracted with phenylephrine (Phe) was treated with four different concentrations of quercetin (10−7, 10−6, 10−5 and 10−4 M). PCCSM were preincubated with N-Nitro-L-arginine methyl ester hydrochloride (L-NAME) and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) to block nitric oxide synthase and guanylate cyclase, respectively. The changes in PCCSM tension were recorded, and cyclic nucleotides in the perfusate were measured by radioimmunoassay. The interactions of quercetin with phosphodiesterase type 5 inhibitors (PDE5-Is) such as sildenafil, udenafil and mirodenafil, were also evaluated. PCCSM relaxation induced by quercetin occurred in a concentrationdependent manner. The application of quercetin to PCCSM pre-treated with L-NAME and ODQ significantly inhibited the relaxation. Quercetin significantly increased cGMP in the perfusate. Furthermore, quercetin enhanced PDE5-Is-induced relaxation of PCCSM. Quercetin relaxed the PCCSM by activating the NO-cGMP signaling pathway, and it may be a therapeutic candidate or an alternative treatment for patients with erectile dysfunction who do not completely respond to PDE5-Is.
Go to : 
References
(1). Kouidrat Y.., Zaitouni A.., Amad A.., Diouf M.., Desailloud R.., Loas G.., Lalau J. D. J.Diabetes Complicat. 2017. 31:108–113.
(2). Sheweita S. A.., Wally M.., Hassan M.Oxid. Med. Cell Longev. 2016. 2016:1–9.
(3). Matos G.., Hirotsu C.., Alvarenga T. A.., Cintra F.., Bittencourt L.., Tufik S.., Andersen M. L.Andrology. 2013. 1:872–878.
(4). Hatzichristou D.., Rosen R. C.., Broderick G.., Clayton A.., Cuzin B.., Derogatis L.., Litwin M.., Meuleman E.., O'Leary M.., Quirk F.., Sadovsky R.., Seftel A. J.Sex. Med. 2004. 1:49–57.
(5). Chiou W. F.., Chen C. F.Eur. J. Pharmacol. 2002. 446:151–159.
(6). Sullivan M. E.., Thompson C. S.., Dashwood M. R.., Khan M. A.., Jeremy J. Y.., Morgan R. J.., Mikhailidis D. P.Cardiovasc. Res. 1999. 43:658–665.
(7). Deng W.., Bivalacqua T. J.., Hellstrom W. J. G.., Kadowitz P. J.Int. J. Impot. Res. 2005. 17:S57–S63.
(8). Yan L.., Zhang J. D.., Wang B.., Lv Y. J.., Jiang H.., Liu G. L.., Qiao Y.., Ren M.., Guo X. F.PLoS One. 2013. 8:1–14.
(9). Bhutada P.., Mundhada Y.., Bansod K.., Bhutada C.., Tawari S.., Dixit P.., Mundhada D.Neurobiol. Learn. Mem. 2010. 94:293–302.
(10). Ranawat P.., Pathak C. M.., Khanduja K. L.Phytother. Res. 2013. 27:802–810.
(11). Senggunprai L.., Kukongviriyapan V.., Prawan A.., Kukongviriyapan U.Phytother. Res. 2014. 28:841–848.
(12). Erden Inal M.., Kahraman A.Toxicology. 2000. 154:21–29.
(13). Chen C. K.., Pace-Asciak C. R.Gen. Pharmacol. 1996. 27:363–366.
(14). Zhao C.., Chae H. J.., Kim S. H.., Cui W. S.., Lee S. W.., Jeon J. H.., Park J. K. J.Sex. Med. 2010. 7:1419–1428.
(15). Cui X.., Lee S. J.., Kim S. Z.., Kim S. H.., Cho K. W.Eur. J. Pharmacol. 2000. 402:129–137.
(16). Li X.., Oh H. C.., Son S. B.., Lee Y. J.., Kang D. G.., Lee H. S.Evid. Based Complement. Alternat. Med. 2012. 2012:1–10.
(17). Feng X. T.., Qin C. B.., Leng J.., Tang Q. L.., Shi H.., Zhai L. N.., Li S. L.Biosci. Biotechnol. Biochem. 2012. 76:257–263.
(18). Nimmegeers S.., Sips P.., Buys E.., Decaluwé K.., Brouckaert P.., Van de Voorde J.Int. J. Impot. Res. 2008. 20:278–284.
(19). Eardley I.Br. J. Diabetes Vasc. Dis. 2002. 2:272–276.
(20). Hosogai N.., Takakura S.., Manda T.., Mutoh S.Eur. J. Pharmacol. 2003. 473:65–70.
(21). Choi B. R.., Kumar S. K.., Zhao C.., Zhang L. T.., Kim C. Y.., Lee S. W.., Jeon J. H.., Soní K. K.., Kim S. H.., Park N. C.., Kim H. K.., Park J. K.Int. J. Impot. Res. 2015. 27:225–232.
Go to : 
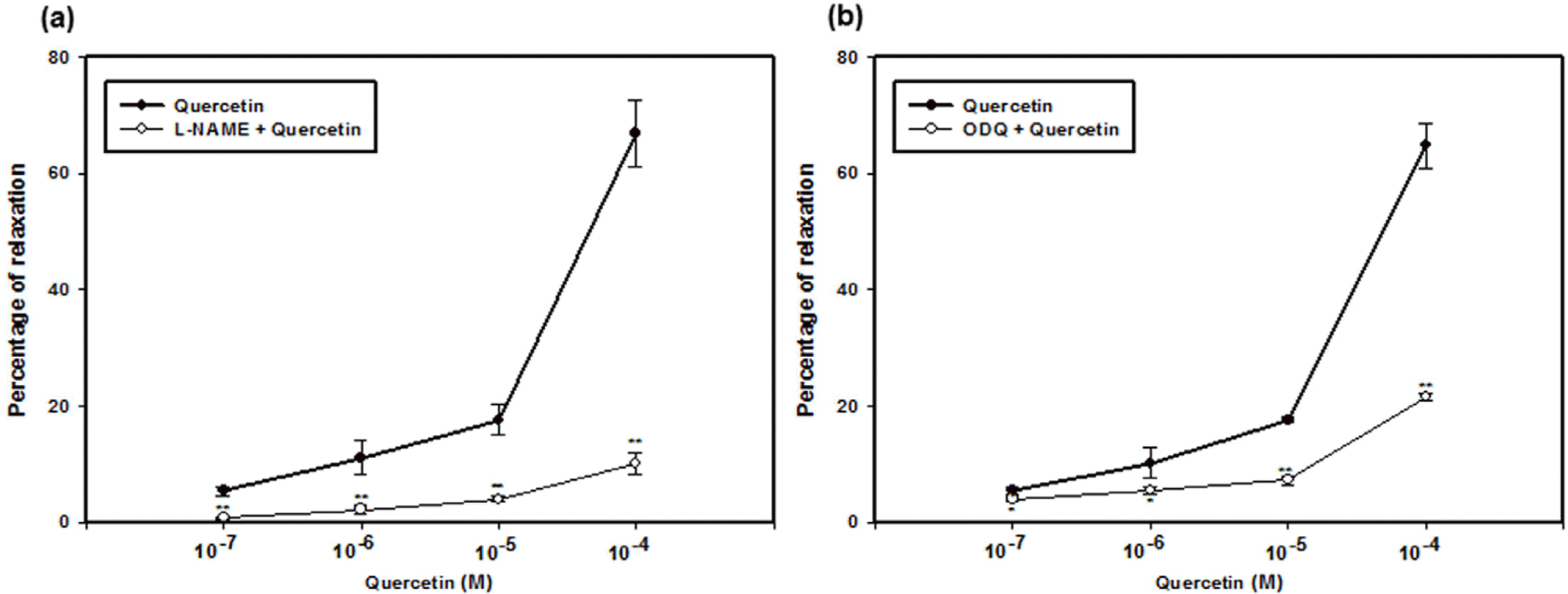 | Fig. 2.Relaxation effect of quercetin in L-phenylephrine (Phe)-induced contraction (n = 4). PCCSM contracted by Phe (10−5 M) that was preincubated with N-Nitro-L-arginine methyl ester hydrochloride (L-NAME, 10−3 M) (a) or 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, 10−5 M) (b) was treated with four concentrations of quercetin (10−7, 10−6, 10−5 and 10−4 M). The submaximal penile contractile responses induced by Phe were taken as the 100% values, and all subsequent responses to quercetin were expressed as a percentage of this value. Each point represents the mean ± SD of the percentages. Statistical analysis was carried out by ANOVA, followed by Bonferroni's test (∗p < 0.05 compared to quercetin, ∗∗p < 0.01 compared to quercetin). |
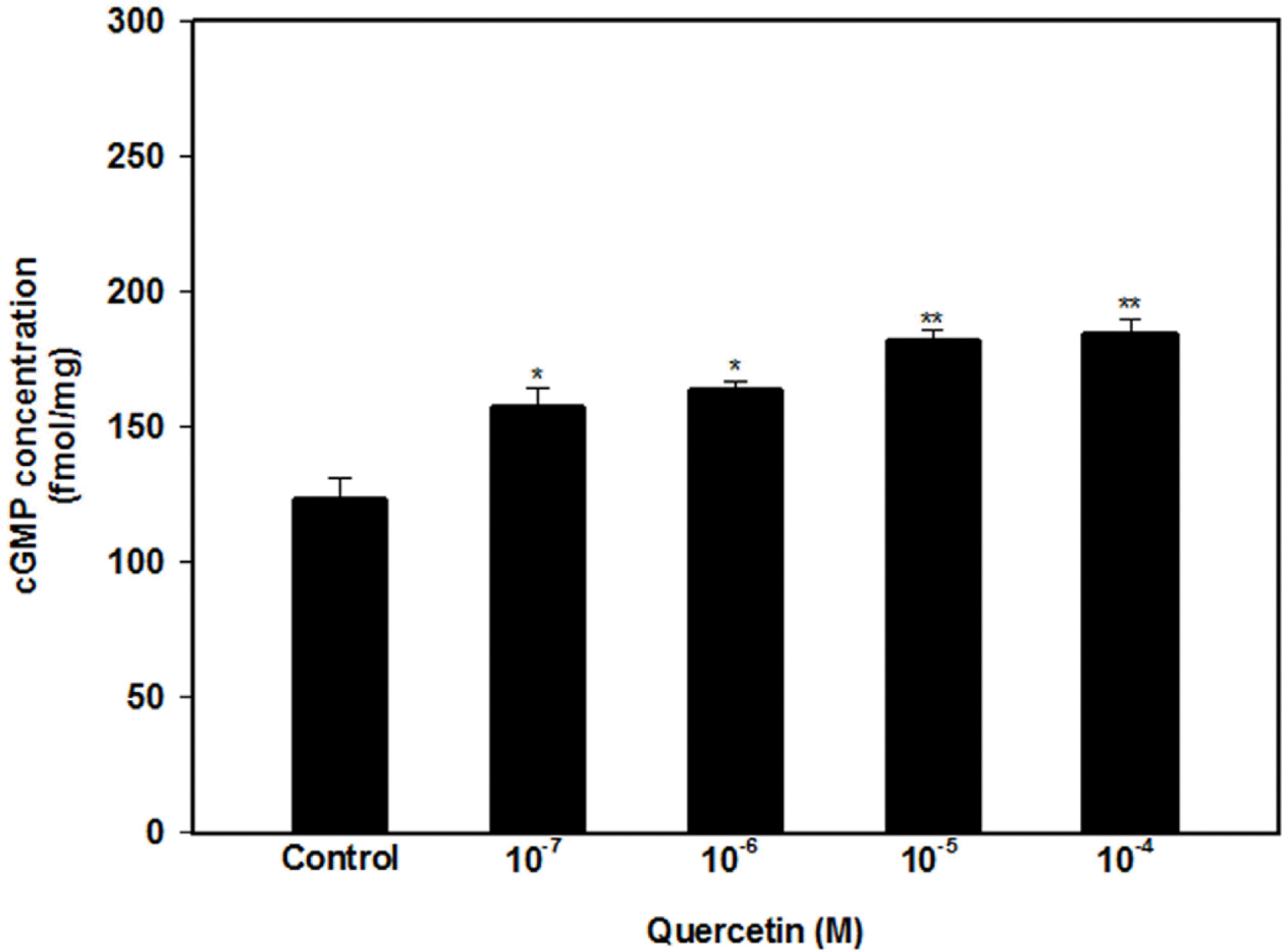 | Fig. 3.Effect of quercetin (10−7, 10−6, 10−5 and 10−4 M) on the cGMP level in the perfusate. Each point represents the mean ± SD of the percentages. Statistical analysis was carried out by ANOVA, followed by Bonferroni's test (∗p < 0.05 compared to control, ∗∗p < 0.01 compared to control). |
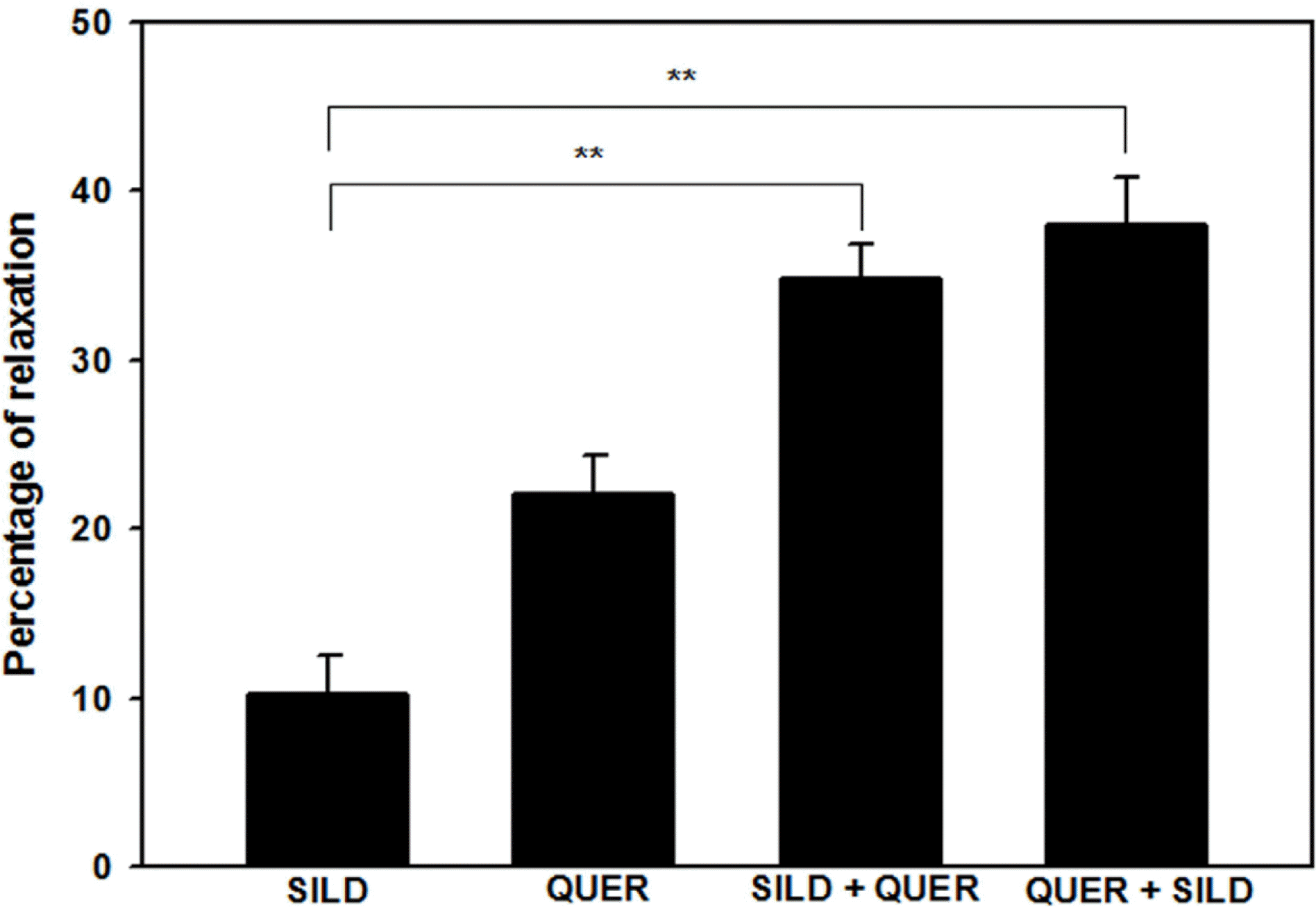 | Fig. 4.Interaction of quercetin (QUER, 10−5 M) with sildenafil (SILD, 10−8 M) (n=4). SILD+QUER indicates quercetin-induced relaxation in sildenafil citrate-preincubated PCCSM. QUER + SILD indicates sildenafil citrate-induced relaxation in QUER-preincubated PCCSM. Each point represents the mean ± SD of percentages of maximal relaxation of the preceding submaximal contractile responses. Statistical analysis was carried out by ANOVA, followed by Bonferroni's test (∗∗p < 0.01 compared to SILD). |
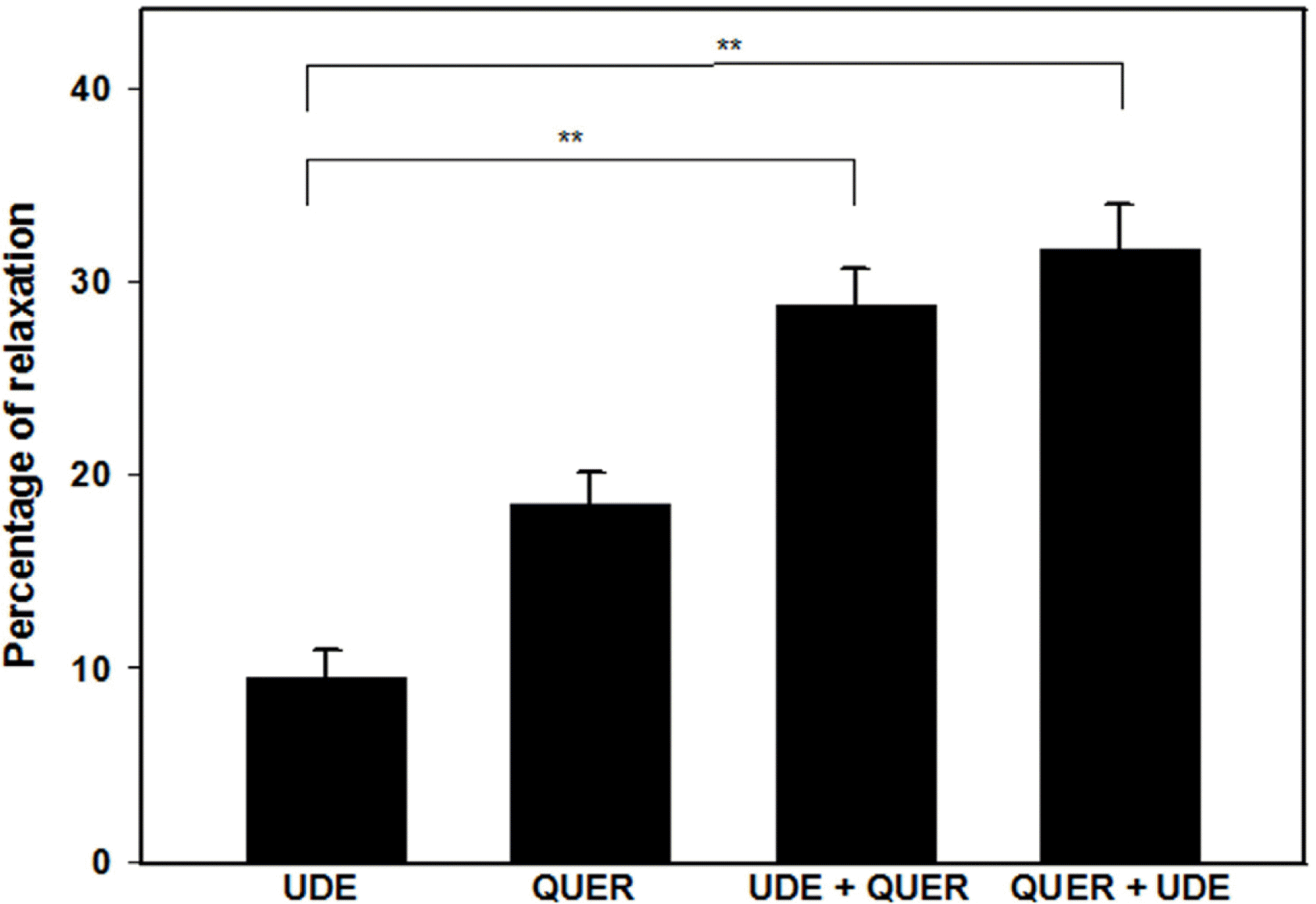 | Fig. 5.Interaction of quercetin (QUER, 10−5 M) with udenafil (UDE, 10−7 M) (n=4). UDE+QUER indicates quercetin-induced relaxation in udenafil-preincubated PCCSM. QUER+UDE indicates udenafil-induced relaxation in QUER-preincubated PCCSM. Each point represents the mean ± SD of percentages of maximal relaxation of the preceding submaximal contractile responses. Statistical analysis was carried out by ANOVA, followed by Bonferroni's test (∗∗p < 0.01 compared to UDE). |
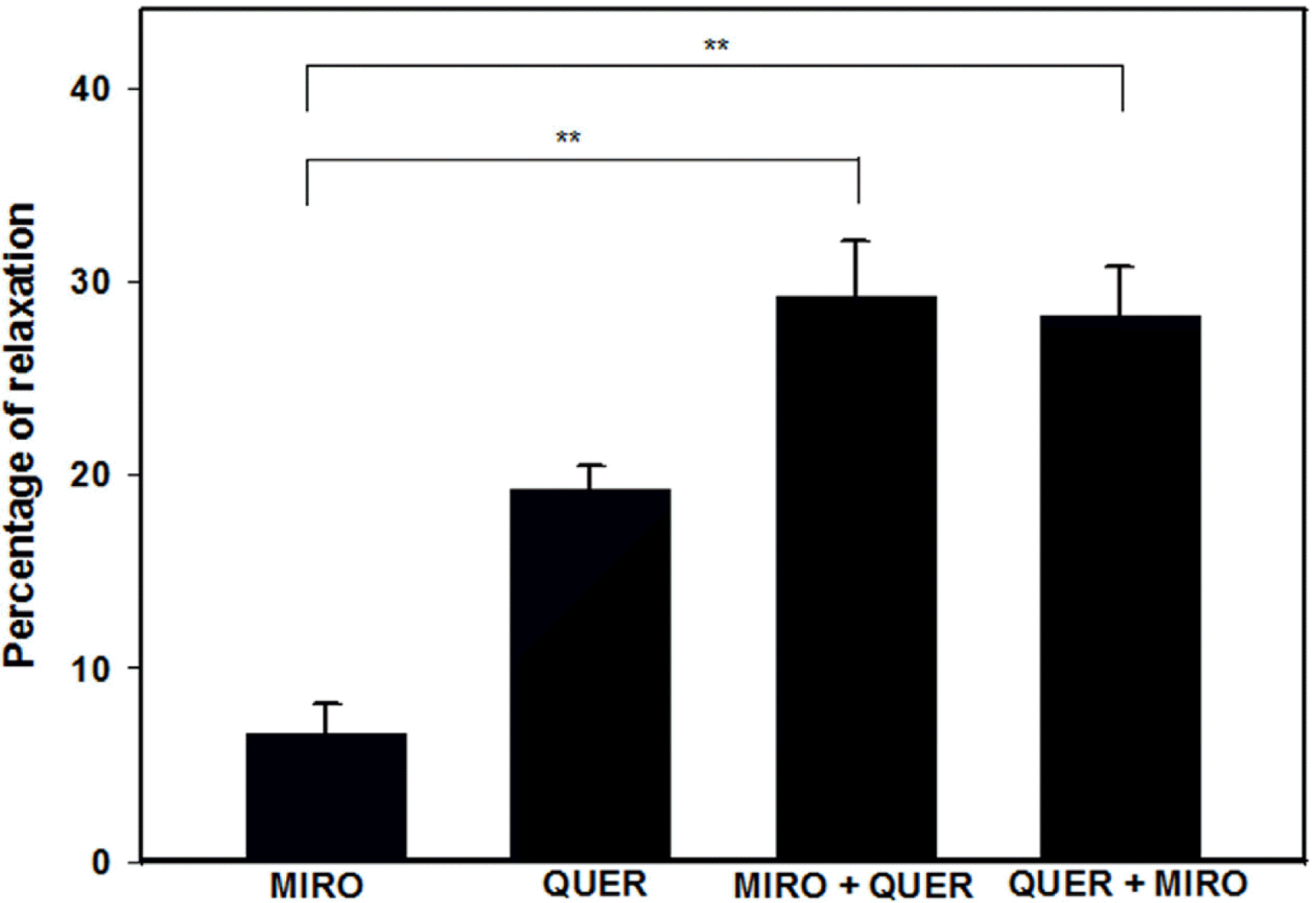 | Fig. 6.Interaction of quercetin (QUER, 10−5 M) with mirodenafil (MIRO, 10−8 M) (n=4). MIRO+QUER indicates quercetin-induced relaxation in mirodenafil hydrochloride-preincubated PCCSM. QUER+MIRO indicates mirodenafil hydrochloride-induced relaxation in QUER-preincubated PCCSM. Each point represents the mean ± SD of percentages of maximal relaxation of the preceding submaximal contractile responses. Statistical analysis was carried out by ANOVA, followed by Bonferroni's test (∗∗p < 0.01 compared to MIRO). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download